
TOWARDS PERSONALIZED DRUG DELIVERY
Preparation of an Encapsulated Multicompartment System
Maik Hadorn
Department of Informatics, Artificial Intelligence Laboratory, University of Zurich, Zurich, Switzerland
Peter Eggenberger Hotz
The Mærsk Mc-Kinney Møller Institute, University of Southern Denmark, Odense M, Denmark
Keywords: Personalized healthcare, Drug delivery, Encapsulation, Compartmentalization, Vesicle, Liposome.
Abstract: Single liposomes and vesicles are successfully utilized as delivery vehicles of pharmaceuticals. However
limitations of these unilamellar, single compartments led to the development of encapsulated multicom-
partment systems that establishes the prospect of multicomponent or multifunctional drug delivery systems.
So far compartmentalization is restricted to binary systems. To realize a personalized drug delivery, a pro-
grammable linkage of n-entities of different content will be needed. Here we present both a programmable
DNA-mediated linkage of three distinct vesicle populations and a novel encapsulation protocol. We discuss
how the techniques established in this study might be used in personalized healthcare based on custom-
tailored encapsulated multicompartment vesicular drug delivery systems.
1 INTRODUCTION
Biological as well as artificial vesicles feature an
aqueous compartment partitioned off an aqueous
surrounding by a lipid membrane that is nearly im-
permeable for hydrophilic substances. The mem-
brane organizes processes by compartmentalizing
them. The compartmentalization enables segregation
of specific chemical reactions for the purposes of
increased controllability, observability, stability, and
biochemical efficiency by restricted dissemination
and efficient storage of reactants, and/or reaction
products. Thus, wide usage of artificial vesicles is
found in analytics (Hotani, Nomura and Suzuki,
1999; Jesorka and Orwar, 2008; Limozin, Roth and
Sackmann, 2005; Luisi and Walde, 2000) and syn-
thetics, where they are used as bioreactors (Bolinger,
Stamou and Vogel, 2008; Michel et al., 2004;
Noireaux and Libchaber, 2004), and drug delivery
systems (Allen and Cullis, 2004; Bonacucina, Cespi,
Misici-Falzi and Palmieri, 2009; Torchilin, 2005).
Vesicles featuring biocompatibility, biodegradabil-
ity, low toxicity, and structural variability are suc-
cessfully utilized as therapeutic agents for the deliv-
ery of antibacterial, antiviral, and anticancer drugs,
as well as of hormones, enzymes, and nucleotides
(Eckstein, 2007; Lasic, Vallner and Working, 1999;
Weissig, Boddapati, Cheng and D'souza, 2006).
Generally, single unilamellar vesicles apply in
therapeutic systems. However premature content
release in physiological environments limits their
reliability (Bakker-Woudenberg, Schiffelers, Storm,
Becker and Guo, 2005). Extending the circulation
time of vesicles that results in accumulation at tu-
mors or inflammation sites due to the enhanced
permeability and retention (EPR) effect (Allen and
Cullis, 2004) is realized at the molecular level via
monomer design (Torchilin, 2009) or at the
mesoscopic level via encapsulation. The bilayer-
within-a-bilayer structure of encapsulated vesicles
not only prevents a premature degradation and con-
tent release (Boyer and Zasadzinski, 2007) but offers
a division of different membrane functions (biocom-
patibility, cargo release, targeting, and protection)
among several membranes of different compositions
and dimensions. Encapsulated vesicles are fre-
quently used in pharmaceutical and cosmetic appli-
cations (Lasic, 1993). The applicability of single
vesicles is limited further by the need for a simulta-
neous entrapment of a given set of (pharmaceutical)
components in one single compartment, which is
5
Hadorn M. and Eggenberger Hotz P. (2010).
TOWARDS PERSONALIZED DRUG DELIVERY - Preparation of an Encapsulated Multicompartment System.
In Proceedings of the Third International Conference on Biomedical Electronics and Devices, pages 5-12
DOI: 10.5220/0002691400050012
Copyright
c
SciTePress
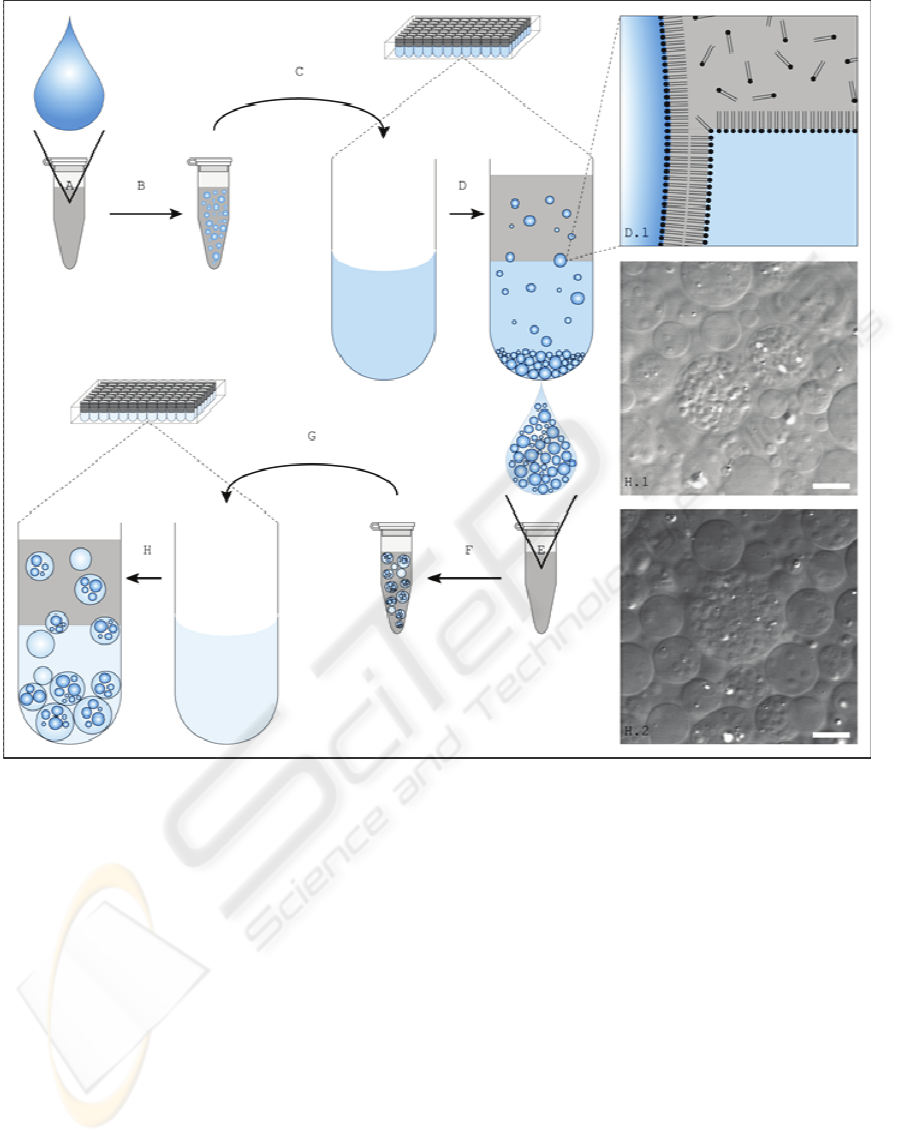
Figure 1: Schematic representation of the vesicle formation/encapsulation procedure and micrographs of internally com-
partmentalized vesicles. (A) A water droplet (blue) is added to a phospholipid suspension (light gray, cp. D.1). (B) A water-
in-oil emulsion is produced by mechanical agitation and sonication. (C) The emulsion is placed over an aqueous solution.
Vesicles are produced in 96-well microtiter plates, providing parallel formation of up to 96 distinct vesicle populations. (D)
Induced by centrifugation, the droplets pass the oil/water interface. Due to the density difference of the inter- and intrave-
sicular fluid and the geometry of the microplate bottom, vesicles pelletize in the centre of the well and become easily acces-
sible for pipetting. (D.1) Amphiphilic phospholipids, solved in mineral oil, stabilize water-oil interfaces by forming
monolayers. Two monolayers form a bilayer when a water droplet passes the interface. (E) A droplet of the aqueous solu-
tion that hosts the vesicles is added to a phospholipid suspension. After mechanical agitation (F) and placing over an aque-
ous solution (G), internally compartmentalized vesicles are produced by centrifugation. Lower color saturation indicates
lower density of the aqueous solution. (H.1, H.2) Differential interference contrast micrographs of internally compartmen-
talized vesicles. Scale bar represents 10μm.
“not an easy matter” (Luisi, de Souza and Stano,
2008, p. 14660). Multicompartment systems can
overcome this limitation by conciliating smaller
subsets of components entrapped in different com-
partments. Thus, encapsulated multicompartment
systems could provide stable vehicles for a multi-
component or multifunctional drug delivery.
Zasadzinski et al. established a protocol to en-
capsulate a multicompartment system of tethered
liposomes (Boyer and Zasadzinski, 2007; Kisak,
Coldren, Evans, Boyer and Zasadzinski, 2004;
Walker, Kennedy and Zasadzinski, 1997). Both
tethering and encapsulation of these vesosomes are
based on the molecular recognition process of the
biotin-streptavidin complex. Like most of the current
tethering strategies (Berti, Baglioni, Bonaccio, Bar-
sacchi-Bo and Luisi, 1998; Chiruvolu et al., 1994;
Constable, Meier, Nardin and Mundwiler, 1999;
BIODEVICES 2010 - International Conference on Biomedical Electronics and Devices
6
Marchi-Artzner et al., 2001; Menger, Seredyuk and
Yaroslavov, 2002; Paleos, Sideratou and Tsiourvas,
1996; Sideratou et al., 2002; Vermette, Taylor, Dun-
stan and Meagher, 2002; Weikl, Groves and Li-
powsky, 2002), tethering is based on single ligand-
receptor pairs and result in systems binary at most.
DNA-mediated linkage (Beales and Vanderlick,
2007; Chan, van Lengerich and Boxer, 2009) offers
multiple ligand-receptors, as needed in a multicom-
ponent or multifunctional drug delivery system.
However neither a coupling of more than two vesi-
cle populations is realized, nor a procedure to encap-
sulate such a system is established.
In this study, we present both a programmable
DNA-mediated linkage of three distinct vesicle
populations and a novel encapsulation mechanism.
Based on the results of this study, we formulate a
scenario how encapsulated multicompartment sys-
tems might be used to realize custom-tailored ve-
sicular drug delivery systems.
2 MATERIALS AND METHODS
Technical modifications of the vesicle formation
protocol reported by Pautot et al. (Pautot, Frisken
and Weitz, 2003) were: (i) the introduction of 96-
well microtiter plates U96 to increase procedural
manageability in laboratory experimentation and (ii)
a density difference between inter- and intravesicu-
lar solution induced by isomolar solutions of mono-
saccharids (glucose: inter) and disaccharids
(sucrose: intra). For a description of the modified
vesicle protocol see Figure 1A-D. For the membrane
composition of the vesicles used in the encapsulation
and the self-assembly experiments see Table 1. All
phospholipids were solved in mineral oil.
Table 1: Membrane composition of vesicles used in ex-
perimentation.
E
ncapsulation
100%
PC(16:0/18:1(Δ9-Cis)) =
1-Palmitoyl-2-Oleoyl-sn-Glycero-3-Phosphocholine
Self-Assembly
99%
PC(16:0/18:1(Δ9-Cis)) =
1-Palmitoyl-2-Oleoyl-sn-Glycero-3-Phosphocholine
0.75%
methyl-PEG2000-PE(18:0/18:0) =
1,2-Distearoyl-sn-Glycero-3-Phosphoethanolamine-N-
[Methoxy (Polyethylene glycol)-2000]
0.25%
biotin-PEG2000-PE(18:0/18:0) =
1,2-Distearoyl-sn-Glycero-3-Phosphoethanolamine-N-
[Biotinyl (Polyethylene Glycol) 2000]
For details of the encapsulation procedure of
untethered vesicles see Figure 1. Encapsulated vesi-
cles exhibited quick random motion within the
boundaries of the surrounding vesicle.
In the compartmentalization experiments, three
distinct vesicle populations were prepared by doping
their surface with binary combinations of six ssDNA
populations (1
st
population: α, β; 2
nd
: α’, γ; 3
rd
: β’,
γ’; for the sequence of biotinylated ssDNA strands
see Table 2). The DNA strands were biotin-labeled
and anchored to biotinylated vesicular membrane via
streptavidin as a cross-linking agent. For a detailed
protocol of the surface doping and the self-assembly
procedure see Figure 2. The sequences of the ssDNA
were produced by a genetic algorithm and optimized
for minimal DNA-DNA-hybridization among the
three pairs. The specificity was verified on a com-
mercial program.
Light and confocal laser scanning microscopy
was performed using an inverted Leica DMR IRE2
SP2 confocal laser scanning microscope.
Table 2: Sequence of complementary biotinylated DNA
single strands used in the self-assembly experiments.
α biotin-TGTACGTCACAACTA-3’
α’ 3’-ACATGCAGTGTTGAT-biotin
β biotin-AGAAAGAGCCCTCCA-3’
β’ 3’-TCTTTCTCGGGAGGT-biotin
γ biotin-AAAGATTACACACGA-3’
γ’ 3’-TTTCTAATGTGTGCT-biotin
3 RESULTS
The density difference between the inter- and in-
travesicular solution induced vesicle pelletization at
the centre of the well. The size distribution of vesi-
cles produced in the first round of vesicle formation
(Figure 1 A-D) was shifted to the left when com-
pared to vesicles produced in the second round (Fig-
ure 1 E-H). The two vesicle formation protocols
differed only in the presence (Figure 1 B) or absence
(Figure 1 F) of the sonication of the water-in-oil
emulsion. To indicate independence of the tethering
and encapsulation process, vesicles to be encapsu-
lated were not tethered. Tethered assemblies were
encapsulated without any modification of the encap-
sulation procedure (results not shown). As seen in
Figure 1 H.1 and H.2 the ratio of vesicles internally
compartmentalized to vesicles uncompartmentalized
was high. Most of the vesicles produced in the first
round were found to be enclosed – encapsulation
efficiency was high.
TOWARDS PERSONALIZED DRUG DELIVERY - Preparation of an Encapsulated Multicompartment System
7
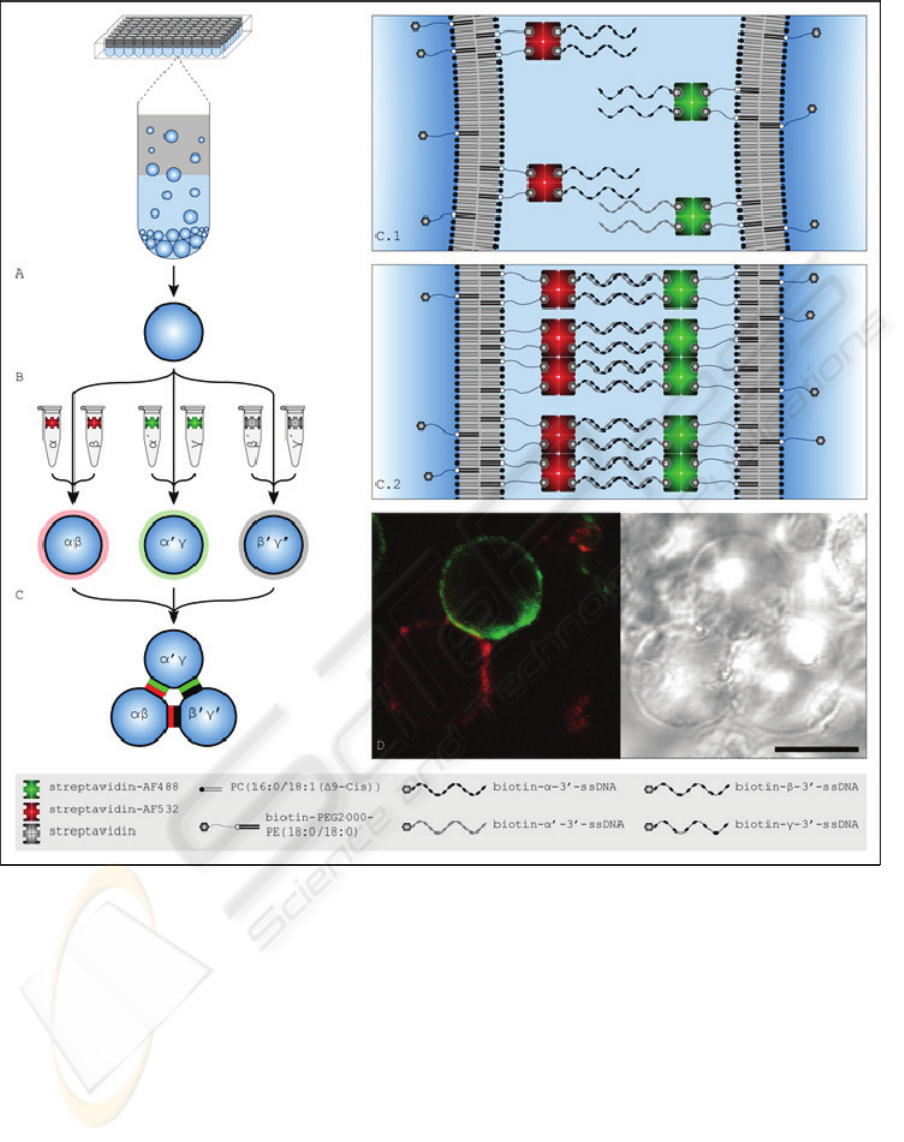
Figure 2: Schematic representation of the self-assembly process and micrographs of adhesion plaques. (A) For vesicle
formation see Figure 1.(B) Vesicle populations become distinct by incubating them with single stranded DNA (ssDNA) of
different sequence (α, α’, β, β’, γ, γ’) and streptavidin differing in fluorescence labeling (Alexa Fluor 488 / 532 conjugate
(AF488 / AF532) or unlabeled). Monohomophilic oligonucleotide-doping of streptavidin is provided by separated incuba-
tions. (C) The vesicle populations are merged. (C.1) The lateral distribution of linkers in the lipid membrane is homogene-
ous. Vesicles doped with complementary ssDNA come into contact. (C.2) Hybridization of DNA strands results in double
stranded DNA and induces the assembly process. Due to their lateral mobility, linkers accumulate in the contact zone form-
ing an adhesion plaque – the lateral distribution of linkers in the outer leaflet becomes inhomogeneous (situation shown for
α-AF532, α’-AF488). (D) CLSM (confocal laser scanning microscope) and DIC (differential interference contrast) micro-
graph of a vesicular aggregate that emerged in real-world experimentation. Accumulation and depletion of linkers are
clearly visible in the CLSM micrograph. Scale bar represents 10μm.
When vesicles doped with complementary ssDNA
came into contact, hybridization of single DNA
strands resulted in double stranded DNA. Linkers
accumulated in the contact area of the two vesicles
formed an adhesion plaque (Figure 2 D). Adhesion
plaques were found exclusively, when DNA strands
were complementary and inorganic ions were pre-
sent (data of control experiments not shown). No
transfer of linkers between the membranes of differ-
ent vesicles was observed (data not shown).
BIODEVICES 2010 - International Conference on Biomedical Electronics and Devices
8

4 DISCUSSION
Multicomponent or multifunctional custom-tailored
vesicular drug delivery systems have to fulfil several
requirements: (i) the actual drug containing system
should be encapsulated to prevent premature
degradation and content release, (ii) the drug
containing system should consist of more than two
distinct compartments, and (iii) the proper
composition of the drug containing system should be
controlled.
4.1 Encapsulation
The in vitro vesicle formation procedure (Noireaux
and Libchaber, 2004; Pautot et al., 2003; Träuble
and Grell, 1971) enables independent tailoring of
chemical material properties of the inter- and in-
travesicular fluid as well as of the inner and outer
membrane leaflet composition. To our knowledge,
the entrapment efficiency of this vesicle formation
procedure is not analyzed so far. However one may
speculate that its entrapment efficiency is better than
for vesicle formation procedures currently used (for
an overview of the current vesicle formation proce-
dures see Jesorka and Orwar (2008)). The potential
of an asymmetric leaflet composition was exempli-
fied by the production of phospholipid and polymer
hybrids combining biocompatibility and mechanical
endurance in single vesicles (Pautot et al., 2003). We
increased procedural manageability of the formation
procedure by introducing microtiter plates and vesi-
cle pelletization (due to density differences in the
inter- and intravesicular fluid). By introducing soni-
cation of the water-in-oil emulsion, we could shift
the size distribution of the vesicles formed. By re-
feeding the vesicle containing solution, we estab-
lished a novel method to produce multivesicular
assemblies. The protocol provides encapsulation of
either tethered or untethered vesicular assemblies.
The interdependence of tethering and encapsulation,
faced in vesosome formation, is therefore resolved.
4.2 Compartmentalization
Single stranded DNA provides programmability,
specificity, and high degrees of complexity (Licata
and Tkachenko, 2006). Streptavidin offers the
strongest noncovalent biological interaction known
(Green, 1990), an extensive range of possible vesicle
modifications, component modularity, and availabil-
ity off the shelf. Phospholipid-grafted biotinylated
PEG tethers feature lateral mobility (Singer and
Nicolson, 1972), high detachment resistance (Bur-
ridge, Figa and Wong, 2004), and no intermembrane
transfer of linkers. The combination of phosphol-
ipid-grafted biotinylated PEG tethers and strepta-
vidin allows fast production of vesicles differently
doped and avoids problems encountered in other
approaches using cholesterol-tagged DNA to spe-
cifically link different vesicle populations by the
hybridization of membrane-anchored DNA (Beales
and Vanderlick, 2007; Benkoski and Hook, 2005;
Chan et al., 2009): (i) Because the processes of vesi-
cle formation and vesicle modification are not sepa-
rated (the cholesterol-tagged ssDNA has to be pre-
sent during vesicle formation), the formation proce-
dure has to be adjusted anew for each change in the
vesicle modification. The procedural manageability
in laboratory experimentation is therefore reduced.
(ii) As discussed by Beales and Vanderlick (2007)
the cholesterol anchors of the cholesterol-tagged
ssDNA spontaneously leave the lipid bilayer and
incorporate randomly into (other) lipid bilayers.
Thus, the specificity of the linking system is lost
over time.
We presented a DNA-mediated tethering of three
distinct vesicle populations. Linkage of more than
two distinct vesicle populations is realized for the
first time. Thus, restriction to binarism faced in
current donor-acceptor mechanisms is resolved. The
DNA-mediated linkage mechanism offers program-
mability of composition of multicompartment sys-
tems. Thus, custom-tailored vesicular drug delivery
systems seem feasible.
4.3 Composition Control
By loading the vesicular membranes of tethered
assemblies by ligand groups not used in the aggrega-
tion process, a column chromatographic purification
procedure of aggregates may be realized. The ligand
groups would be used to purify aggregates from
single vesicles (for details see Figure 3). Figure 3
depicts the minimal situation of tethered assemblies
of two vesicle populations and two columns in se-
ries. If the tethered assemblies consist of three dif-
ferent vesicle populations bearing three different
ligand groups not used in the aggregation process,
purification of aggregates of proper composition
both from single vesicles and incomplete aggregates
might become possible.
By a downstream fluorescence activated cell
sorting (FACS; for a review of techniques used in
cell separation see Pappas and Wang (2007)) inter
nally compartmentalized vesicles might be purified
from vesicles not equipped properly (Figure 3G).
Based on the fluorescence signal, the chroma-
tographic separation procedure (Figure 3 B-D) might
TOWARDS PERSONALIZED DRUG DELIVERY - Preparation of an Encapsulated Multicompartment System
9
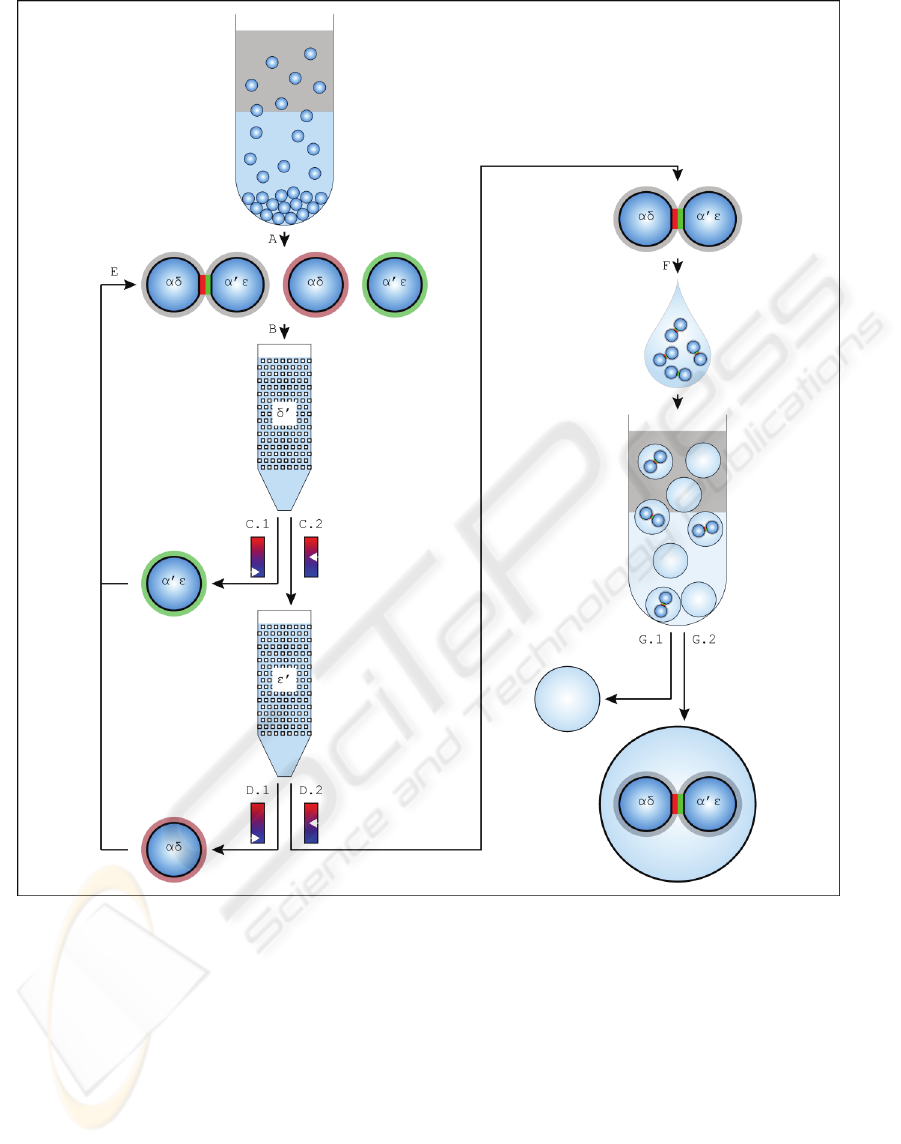
Figure 3: Schematic representation of the processes to form and purify internally compartmentalized vesicles. (A) For de-
tails concerning the self-assembly process resulting in vesicular aggregates see Figure 2. Vesicle populations become dis-
tinct by incubating them with single stranded DNA (ssDNA) of different sequence (α, α’, δ, ε) and streptavidin differing in
fluorescence labeling (Alexa Fluor 488 (green) / 532 (red) conjugate (AF488 / AF532) or unlabeled (black)) resulting in a
surface doping of (α-AF532, δ-unlabeled; α’-AF488, ε-unlabeled). The DNA strands differ in their melting temperature (T
> T
= T
). Due to the hybridization of DNA strands linkers α-AF532 and α’-AF488 accumulate in the contact zone form-
ing an adhesion plaque. Lateral distribution of linkers δ-unlabeled and ε-unlabeled is not affected by the assembly process.
(B) To dispose vesicles not assembled the mixture is fed to a column whose stationary phase is doped with single stranded
DNA (δ’, ε’). Hybridization of DNA strands results in a retention of aggregates and single vesicles doped with ssDNA (δ).
(C.1) Single vesicles not doped with ssDNA (δ) are eluted at low temperature (T < T
, T
, T
). (C.2) An increase in
temperature (T
, T
< T < T
) results in an elution of aggregates and single vesicles doped with ssDNA (δ). (D.1, D.2)
The procedure of C.1 and C.2 is repeated for a column doped with ε’. (E) Single vesicles can be fed back in the self-
assembly procedure (see Figure 2.C). (F) The purified aggregates are encapsulated in vesicles (for details see Figure 2 E-H).
(G.1, G.2) The fluorescence signal of the internal compartments is exploited to purify internally compartmentalized vesicles
from vesicles not equipped properly.
BIODEVICES 2010 - International Conference on Biomedical Electronics and Devices
10

be replaced by a final FACS for proper composition
of the tethered assemblies. By introducing an inter-
mediate separation process, a feedback of single
vesicles and incomplete assemblies into the self-
assembly process may be realized before they be-
come encapsulated (Figure 3 E). This may increase
encapsulation efficiency and therefore may econo-
mize the production of custom-tailored vesicular
drug delivery systems.
Encapsulation provides an extended circulation
time resulting in accumulation at tumors or inflam-
mation sites due the EPR effect, without the need of
specific targeting. On the other hand, multiple com-
partments offer segregation of multicomponent
pharmaceuticals that might be released only when
and where they are needed. Permeability control
might be realized either by exploitation of stimuli
inherent to target site (pH, redox potential, tempera-
ture) or externally induced (temperature, magnetic
field, ultrasound). For a recent review on stimuli-
sensitive pharmaceutical nanocarriers see Torchilin
(2009).
5 CONCLUSIONS
Encapsulated multicompartment systems may pro-
vide stable vehicles for a multicomponent or multi-
functional personalized drug delivery. In this work,
we established a novel encapsulation technique and
provide evidence for the first stable DNA-mediated
linkage of more than two vesicle populations. We
discussed how these techniques may personalize the
individual healthcare by providing custom-tailored
vesicular drug delivery systems.
ACKNOWLEDGEMENTS
Maik Hadorn was supported by the Swiss National
Foundation Project 200020-118127 Embryogenic
Evolution: From Simulations to Robotic Applica-
tions. Peter Eggenberger Hotz was partly supported
by the European Union integrated project PACE
(EU-IST-FP6-FET-002035). We thank Eva Bönzli
for careful reading of the manuscript.
REFERENCES
Allen, T. M. and Cullis, P. R. (2004). Drug delivery sys-
tems: Entering the mainstream. Science, 303(5665),
1818-1822.
Bakker-Woudenberg, I., Schiffelers, R. M., Storm, G.,
Becker, M. J. and Guo, L. (2005). Long-circulating
sterically stabilized liposomes in the treatment of in-
fections, Liposomes, Pt E (391: 228-260). San Diego:
Elsevier Academic Press Inc.
Beales, P. A. and Vanderlick, T. K. (2007). Specific bind-
ing of different vesicle populations by the hybridiza-
tion of membrane-anchored DNA. Journal of Physical
Chemistry A, 111(49), 12372-12380.
Benkoski, J. J. and Hook, F. (2005). Lateral mobility of
tethered vesicle - DNA assemblies. Journal of Physi-
cal Chemistry B, 109(19), 9773-9779.
Berti, D., Baglioni, P., Bonaccio, S., Barsacchi-Bo, G. and
Luisi, P. L. (1998). Base complementarity and nucleo-
side recognition in phosphatidylnucleoside vesicles.
Journal of Physical Chemistry B, 102(1), 303-308.
Bolinger, P. Y., Stamou, D. and Vogel, H. (2008). An
integrated self-assembled nanofluidic system for con-
trolled biological chemistries. Angewandte Chemie-
International Edition, 47(30), 5544-5549.
Bonacucina, G., Cespi, M., Misici-Falzi, M. and Palmieri,
G. F. (2009). Colloidal Soft Matter as Drug Delivery
System. Journal of Pharmaceutical Sciences, 98(1),1-42.
Boyer, C. and Zasadzinski, J. A. (2007). Multiple lipid
compartments slow vesicle contents release in lipases
and serum. Acs Nano, 1(3), 176-182.
Burridge, K. A., Figa, M. A. and Wong, J. Y. (2004).
Patterning adjacent supported lipid bilayers of desired
composition to investigate receptor-ligand binding un-
der shear flow. Langmuir, 20(23), 10252-10259.
Chan, Y. H. M., van Lengerich, B. and Boxer, S. G.
(2009). Effects of linker sequences on vesicle fusion
mediated by lipid-anchored DNA oligonucleotides.
Proceedings of the National Academy of Sciences of
the United States of America, 106(4), 979-984.
Chiruvolu, S., Walker, S., Israelachvili, J., Schmitt, F. J.,
Leckband, D. and Zasadzinski, J. A. (1994). Higher-
order self-assembly of vesicles by site-specific bind-
ing. Science, 264(5166), 1753-1756.
Constable, E. C., Meier, W., Nardin, C. and Mundwiler, S.
(1999). Reversible metal-directed assembly of clusters
of vesicles. Chemical Communications,(16), 1483-
1484.
Eckstein, F. (2007). The versatility of oligonucleotides as
potential therapeutics. Expert Opinion on Biological
Therapy, 7(7), 1021-1034.
Green, N. M. (1990). Avidin and streptavidin. Methods in
Enzymology, 184, 51-67.
Hotani, H., Nomura, F. and Suzuki, Y. (1999). Giant
liposomes: from membrane dynamics to cell morpho-
genesis. Current Opinion in Colloid & Interface Sci-
ence, 4(5), 358-368.
Jesorka, A. and Orwar, O. (2008). Liposomes: Technolo-
gies and Analytical Applications. Annual Review of
Analytical Chemistry, 1, 801-832.
Kisak, E., Coldren, B., Evans, C., Boyer, C. and Zasadzin-
ski, J. (2004). The vesosome-A multicompartment
drug delivery vehicle. Current medicinal chemistry,
11(2), 199-220.
TOWARDS PERSONALIZED DRUG DELIVERY - Preparation of an Encapsulated Multicompartment System
11

Lasic, D., Vallner, J. and Working, P. (1999). Sterically
stabilized liposomes in cancer therapy and gene deliv-
ery. Current opinion in molecular therapeutics, 1(2),
177.
Lasic, D. D. (1993). Liposomes : from physics to applica-
tions. Amsterdam; New York: Elsevier.
Licata, N. A. and Tkachenko, A. V. (2006). Errorproof
programmable self-assembly of DNA-nanoparticle
clusters. Physical Review E (Statistical, Nonlinear,
and Soft Matter Physics), 74(4), 041406.
Limozin, L., Roth, A. and Sackmann, E. (2005). Microvis-
coelastic moduli of biomimetic cell envelopes. Physi-
cal Review Letters, 95(17), 4.
Luisi, P. and Walde, P. (2000). Giant vesicles. Chichester:
John Wiley & Sons, Ltd.
Luisi, P. L., de Souza, T. P. and Stano, P. (2008). Vesicle
Behavior: In Search of Explanations. Journal of
Physical Chemistry B, 112(46), 14655-14664.
Marchi-Artzner, V., Gulik-Krzywicki, T., Guedeau-
Boudeville, M. A., Gosse, C., Sanderson, J. M.,
Dedieu, J. C. and Lehn, J. M. (2001). Selective adhe-
sion, lipid exchange and membrane-fusion processes
between vesicles of various sizes bearing complemen-
tary molecular recognition groups. Chemphyschem,
2(6), 367-376.
Menger, F. M., Seredyuk, V. A. and Yaroslavov, A. A.
(2002). Adhesive and anti-adhesive agents in giant
vesicles. Angewandte Chemie-International Edition,
41(8), 1350-1352.
Michel, M., Winterhalter, M., Darbois, L., Hemmerle, J.,
Voegel, J. C., Schaaf, P. and Ball, V. (2004). Giant
liposome microreactors for controlled production of
calcium phosphate crystals. Langmuir, 20(15), 6127-
6133.
Noireaux, V. and Libchaber, A. (2004). A vesicle bioreac-
tor as a step toward an artificial cell assembly. Pro-
ceedings of the National Academy of Sciences of the
United States of America, 101(51), 17669-17674.
Paleos, C. M., Sideratou, Z. and Tsiourvas, D. (1996).
Mixed vesicles of didodecyldimethylammonium bro-
mide with recognizable moieties at the interface. Jour-
nal of Physical Chemistry, 100(33), 13898-13900.
Pappas, D. and Wang, K. (2007). Cellular separations: A
review of new challenges in analytical chemistry. Ana-
lytica Chimica Acta, 601(1), 26-35.
Pautot, S., Frisken, B. J. and Weitz, D. A. (2003). Engi-
neering asymmetric vesicles. Proceedings of the Na-
tional Academy of Sciences of the United States of
America, 100(19), 10718-10721.
Sideratou, Z., Foundis, J., Tsiourvas, D., Nezis, I. P.,
Papadimas, G. and Paleos, C. M. (2002). A novel den-
drimeric "glue" for adhesion of phosphatidyl choline-
based liposomes. Langmuir, 18(13), 5036-5039.
Singer, S. J. and Nicolson, G. L. (1972). Fluid mosaic
model of structure of cell-membranes. Science,
175(4023), 720-&.
Torchilin, V. (2009). Multifunctional and stimuli-sensitive
pharmaceutical nanocarriers. European Journal of
Pharmaceutics and Biopharmaceutics, 71(3), 431-444.
Torchilin, V. P. (2005). Recent advances with liposomes
as pharmaceutical carriers. Nature Reviews Drug Dis-
covery, 4(2), 145-160.
Träuble, H. and Grell, E. (1971). Carriers and specificity
in membranes. IV. Model vesicles and membranes.
The formation of asymmetrical spherical lecithin vesi-
cles. Neurosciences Research Program bulletin, 9(3),
373.
Vermette, P., Taylor, S., Dunstan, D. and Meagher, L.
(2002). Control over PEGylated-liposome aggregation
by NeutrAvidin-biotin interactions investigated by
photon correlation spectroscopy. Langmuir, 18(2),
505-511.
Walker, S. A., Kennedy, M. T. and Zasadzinski, J. A.
(1997). Encapsulation of bilayer vesicles by self-
assembly. Nature, 387(6628), 61-64.
Weikl, T. R., Groves, J. T. and Lipowsky, R. (2002).
Pattern formation during adhesion of multicomponent
membranes. Europhysics Letters, 59(6), 916-922.
Weissig, V., Boddapati, S., Cheng, S. and D'souza, G.
(2006). Liposomes and liposome-like vesicles for drug
and DNA delivery to mitochondria. Journal of Lipo-
some Research, 16(3), 249-264.
BIODEVICES 2010 - International Conference on Biomedical Electronics and Devices
12
