
Analysis of Spontaneous MEG Activity in Mild Cognitive Impairment
using the Wavelet Turbulence
Jesús Poza
1
, Carlos Gómez
1
, María García
1
, Alberto Fernández
2
and Roberto Hornero
1
1
Biomedical Engineering Group, Dept. of Signal Theory and Communications, University of Valladolid, Valladolid, Spain
2
Departmento de Psiquiatría y Psicología Médica, Complutense University of Madrid, Madrid, Spain
Keywords: Mild Cognitive Impairment, Magnetoencephalogram, Continuous Wavelet Transform, Wavelet Turbulence,
Irregularity.
Abstract: Mild cognitive impairment (MCI) is usually considered a pre-clinical stage of Alzheimer’s disease (AD).
An appropriate characterization of MCI is crucial to achieve an early diagnosis of AD. Over the last few
years, much effort has been devoted to identifying new diagnostic tests; tough further research is still
required. In this study, we analyzed the spontaneous magnetoencephalographic (MEG) activity from 18
MCI subjects and 27 healthy controls to characterize the irregularity patterns in MCI. For that purpose, the
wavelet turbulence (WT) was calculated from the time-scale representation provided by the continuous
wavelet transform (CWT). Our results revealed that the mean values and the standard deviation of WT for
MCI subjects were significantly higher and lower (p < 0.05) than for controls, respectively. These findings
support the notion that MCI is associated with a significant decrease in irregularity and variability when
compared to normal aging. A Receiver Operating Characteristic (ROC) analysis with a leave-one-out cross-
validation procedure was applied to assess the diagnostic ability of WT. We obtained an accuracy of 66.7%
and an area under ROC curve of 0.704. We conclude that the WT extends the concept of irregularity and
provides potential descriptors of spontaneous MEG activity in MCI.
1 INTRODUCTION
Alzheimer’s disease (AD) is a neurodegenerative
disorder that represents the leading form of dementia
in western countries (Blennow et al., 2006). In order
to optimize treatments, an intervention in early
stages is required, even before the appearance of the
first clinical symptoms (Reitz et al., 2011). For that
purpose, the understanding of mild cognitive
impairment (MCI) is a key point.
MCI is usually considered as an important risk
factor, since the probability of developing AD
among MCI subjects is about 5 to 10 times higher
than for healthy subjects (Petersen and Morris,
2003). No definite diagnostic test is currently
available to detect MCI (Albert et al., 2011).
Therefore, more research is required to address the
gaps in our understanding of MCI.
Scientific evidence suggests that MCI and AD
affect several regions in the cortex (Reitz et al.,
2011).Therefore, brain activity will be modified to
some extent. Electroencephalographic (EEG) and
magnetoencephalographic (MEG) recordings are
useful to describe abnormal neurooscillatory
activity. There exist some differences between EEG
and MEG oscillations. MEG recordings are
reference free and are less affected by the volume
conduction when compared to EEG rhtyms (Stam,
2010). Even some studies suggest that MEG might
be more sensitive to measure the cortical activity
than scalp EEG (Stam, 2010). As a consequence,
they reflect slightly different features (Rampp and
Stefan, 2007).
Several MEG studies have addressed the
characterization of spontaneous brain oscillations in
AD (Stam, 2010). Nevertheless, only a few studies
have focused on MCI. Spectral and non-linear MEG
studies found subtle differences between MCI and
control subjects (Bruña et al., 2012; Escudero et al.,
2011; Fernández et al., 2006, 2010; Gómez et al.,
2009). Their results showed that MCI subjects
exhibit intermediate abnormalities between AD
patients and elderly controls. However, further
efforts are needed to appropriately characterize
abnormal brain dynamics in MCI.
721
Poza J., Gómez C., García M., Fernández A. and Hornero R..
Analysis of Spontaneous MEG Activity in Mild Cognitive Impairment using the Wavelet Turbulence.
DOI: 10.5220/0004182107210726
In Proceedings of the 4th International Joint Conference on Computational Intelligence (SSCN-2012), pages 721-726
ISBN: 978-989-8565-33-4
Copyright
c
2012 SCITEPRESS (Science and Technology Publications, Lda.)

In this study, we applied a new method to
characterize MEG rhythms in MCI. The proposed
parameter is based on the continous wavelet
transform (CWT), which provides an alternative
description to conventional spectral and non-linear
measures. Thus, the wavelet turbulence (WT) was
used to explore the irregularity of MEGs in terms of
the degree of similarity among adjacent wavelet
decompositions. The WT provides an alternative
way to characterize the irregularity in comparison to
previously applied spectral and non-linear methods.
Therefore, the aims of this study were: (i) to analyze
the irregularity patterns based on a new parameter,
(ii) to describe the abnormalities of MCI in
comparison to cognitive decline in normal aging,
and (iii) to introduce an alternative framework to
understand brain dynamics.
2 MATERIALS
2.1 Subjects
MEG signals from forty-five subjects were recorded
at the “Centro de Magnetoencefalografía Dr. Pérez-
Modrego” (Complutense University of Madrid,
Spain). Eighteen subjects (8 men and 10 women, age
= 74.9 ± 5.6 years, mean ± standard deviation M ±
SD) were MCI patients derived from the
“Asociación de Familiares de Enfermos de
Alzheimer”. Diagnoses were made following
Petersen’s criteria (Petersen et al., 2001). The
cognitive and functional deficits were assessed using
the Mini-Mental State Examination (MMSE) and the
Functional Assessment Staging (FAST). MCI
subjects obtained mean scores of 25.7 ± 1.8 and 3.0
± 0.0 on the MMSE and FAST, respectively. None
of these MCI patients suffered from any other
significant medical, neurological or psychiatric
disorder.
Twenty-seven healthy subjects (11 men and 16
women, age = 71.5 ± 6.2 years, M ± SD)
participated in the study as a control group. They
were cognitively normal elderly controls with no
history of neurological or psychiatric disorders.
Mean MMSE and FAST scores were 29.0 ± 1.2 and
1.6 ± 0.5, respectively.
None of the subjects was taking drugs that could
affect MEG activity at the recording time. Mean age
and gender were not significantly different for MCI
subjects and controls (p > 0.05, Mann-Whitney U-
test). All healthy volunteers and caregivers of
patients accepted to participate in the study and gave
their written informed consent. The research was
approved by the Research Ethics Committee of the
center.
2.2 Meg Recording
MEG signals were obtained using a 148-channel
whole-head magnetometer (MAGNES 2500 WH,
4D Neuroimaging, San Diego, CA), placed in a
magnetically shielded room in the “Centro de
Magnetoencefalografía Dr. Pérez Modrego”. During
recordings participants were asked to remain awake,
relaxed and with their eyes closed, in order to
minimize the presence of artifacts. Five minutes of
spontaneous MEG activity were acquired for each
subject with a sampling rate of 678.17 Hz. A 0.1-
200 Hz hardware band-pass filter and a 50 Hz notch
filter were applied. Each MEG recording was
downsampled by a factor of four to reduce the data
length. Artifact-free epochs of 10 s (26.6 ± 5.8
artifact-free epochs per channel and subject, M ±
SD) were selected for further analysis. Prior to
calculation of parameters, MEG signals were
processed using a finite impulse response (FIR) filter
designed with a Hamming window and cut-off
frequencies at 1 and 70 Hz.
3 METHODS
3.1 Continuous Wavelet Transform
Electromagnetic brain signals are non-stationary
biomedical recordings (Blanco et al., 1995). In order
to accurately characterize their spectral time-varying
properties, non-stationary signal analysis techniques
are required. There exist different time-frequency
representations, like the widely used short-time
Fourier transform (STFT). However,
electromagnetic brain signals show high frequency
and short time patterns, or low frequency and long
time oscillations (Figliola and Serrano, 1997).
Therefore, it should be appropriate to use time-
frequency representations with a variable time-
frequency resolution. This is the case of the wavelet
transform, which provides a good time resolution at
high frequencies and a good frequency resolution at
low frequencies (Figliola and Serrano, 1997).
In this study, the time-frequency (or time-scale)
maps were computed for each 10 s MEG epoch
(x(t)) using the CWT with the real Morlet wavelet
as,
,
1
),(
dt
s
kt
tx
s
skCWT
(1)
IJCCI2012-InternationalJointConferenceonComputationalIntelligence
722
where ψ(k) is the “mother” wavelet, s is the scaling
factor and * denotes the complex conjugate. The
wavelet analysis was carried out for scales [1:128] to
include the 1-70 Hz frequency range. Scales 1 and
128 were discarded from analysis, since they are out
of the considered bandwidth.
As previously mentioned, the “mother” wavelet
was the real Morlet wavelet. Previous EEG studies
have recommended the use of the complex Morlet
wavelet (Vialatte et al., 2011). Nevertheless, in this
preliminary analysis we selected the simplest Morlet
wavelet as a first step to characterize the spectral
content of MEG recordings. Its definition can be
read as,
,5cos
2
2
kek
k
(2)
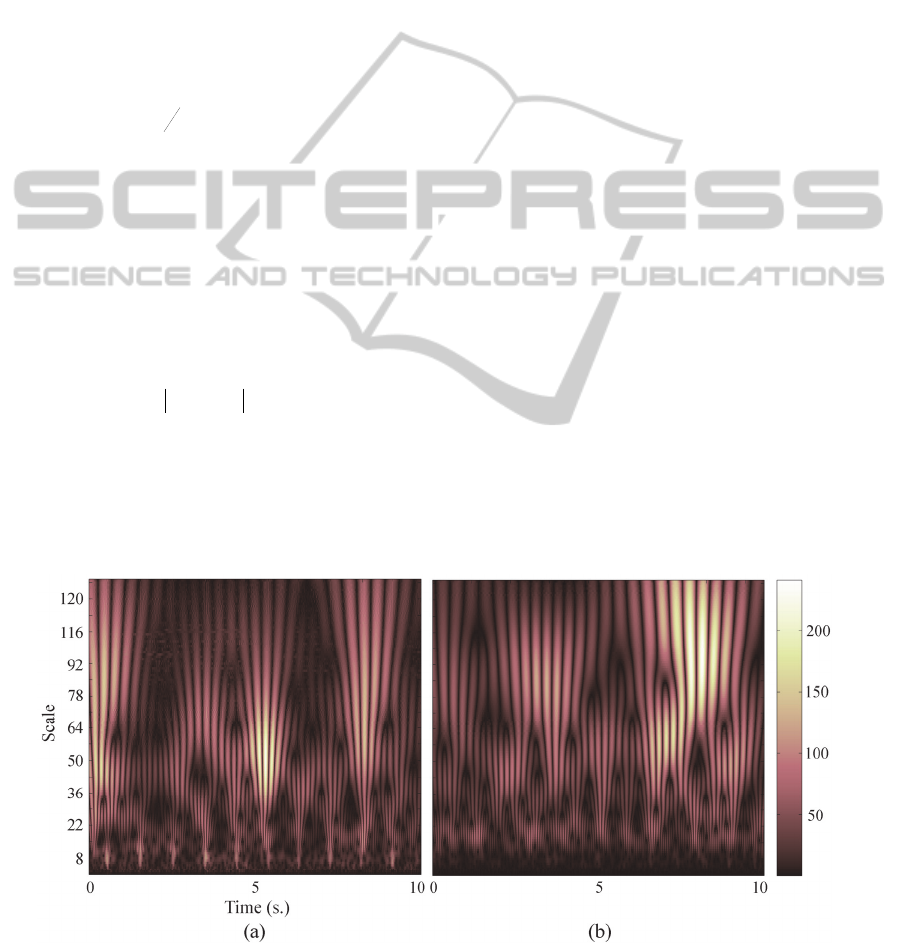
Figure 1 illustrates the time-scale maps for a 10 s
MEG epoch from a healthy control (Figure 1.a) and
a MCI subject (Figure 1.b).
Usually, the wavelet power is computed instead
of the CWT coefficients, as a useful and intuitive
way to analyze the characteristics of a signal. The
so-called wavelet spectrum (WS) or scalogram is a
function that represents the distribution of wavelet
power in the time-scale map (Percival, 1995).
.,,
2
skCWTskWS
(3)
3.2 Wavelet Turbulence
The WT is a parameter that quantifies the spectral
(or scale) changes over time (Kelen et al., 1991).
From
the time-scale map of the WS, the adjacent
wavelet power spectra are compared using the
correlation coefficients (Kelen et al., 1991; Barbosa
et al., 2006).
,,1,, skWSskWSkWT
(4)
where
[] denotes the Kendall correlation between
WS(k,s) and WS(k+1,s). The mean (<WT>) and the
standard deviation (SD[WT]) of WT are calculated
from the time series formed by the Kendall
correlation coefficients (Kelen et al., 1991; Barbosa
et al., 2006). The mean summarizes the average
degree of similarity between the spectral content of
adjacent time slices, whereas the standard deviation
describes the lack of homogeneity in correlation
around the average value (Kelen et al., 1991).
3.3 Statistical Analysis
An exploratory analysis was initially performed to
study the data distribution. Variables did not meet
parametric test assumptions. Hence, non-parametric
Mann-Whitney U-tests were carried out to assess
statistical significance for each parameter ( = 0.05).
A Receiver Operating Characteristic (ROC)
analysis with a leave-one-out cross-validation
procedure (ROC-LOO-CV) was employed to study
diagnostic performance of the parameters.
Classification results were summarized in terms of
sensitivity (Sens.), specificity (Spec.), accuracy
(Acc.) and area under ROC curve (AUC).
The statistical and signal processing analyses
were performed using the software package Matlab
®
(version 7.8.0; Mathworks, Natick, MA).
Figure 1: Absolute wavelet coefficients for an epoch of 10 s obtained from the CWT using the Morlet wavelet (for scales:
[1:128]). (a) Healthy control; (b) MCI subject. CWT coefficients at scales 1 and 128 are zero, since MEG recordings have
been filtered between 1 Hz and 70 Hz.
AnalysisofSpontaneousMEGActivityinMildCognitiveImpairmentusingtheWaveletTurbulence
723
4 RESULTS AND DISCUSSION
We calculated the WS of the 10 s MEG epochs for
the 148 channels. The WT was then computed using
the WS. Finally, mean values (<WT>) and standard
deviations (SD[WT]) of the WT were calculated.
Statistical significance was assessed using Mann-
Whitney U-tests, whereas classification performance
was evaluated by means of a ROC-LOO-CV.
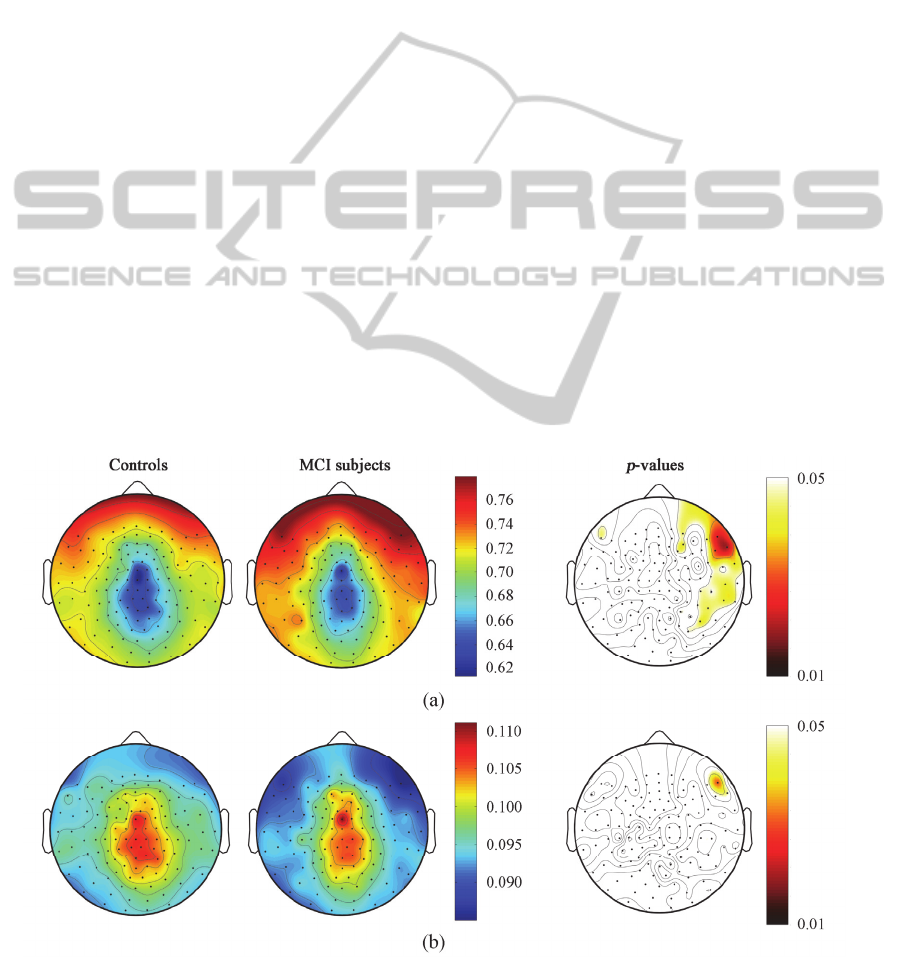
Detailed results for <WT> and SD[WT] values are
shown in Figure 2, where the differences in the
spatial distributions can be observed. Specifically,
the analyses showed a significantly higher <WT>
(right fronto-temporal region, p < 0.05) in MCI
subjects than controls. Likewise, SD[WT] displayed
significant decreases in the right frontal region (p <
0.05), though the differences were more localized
than for <WT>. These results suggest that MEG
background activity in MCI have a significantly
higher degree of similarity and lower variability in
the spectral content than in normal aging. Figure 1
illustrates this issue. Figure 1.a depicts how the
time-scale map from a healthy control is blurred or
distorted (mainly at scales [78:120]) in comparison
to that from a MCI subject (Figure 1.b). Due to the
fact that <WT> is as an indirect measure of the
irregularity in the signal, MCI can be associated with
an irregularity loss in the spontaneous MEG activity.
The decrease in irregularity has also been reported in
previous studies which analyzed spontaneous MEG
activity in MCI using spectral entropies, statistical
disequilibrium (Bruña et al., 2012) and non-linear
parameters (Fernández et al., 2010). Likewise,
similar spatial patterns of significant differences
were observed in a previous MEG study that
reported a lateralization of the reduction in spectral
entropies and statistical disequilibrium (Bruña et al.,
2012). The irregularity decrease can be linked to a
loss in frequency components and, as a consequence,
to a decrease in information content and processing
within the brain cortex (Baraniuk et al., 2003; Poza
et al., 2008a).
Classifications statistics using the ROC-LOO-
CV analysis for <WT> and SD[WT] are summarized
in Table 1. The highest AUC was achieved by <WT>
(0.704, AUC). It is noteworthy that the same
accuracy value (66.7%, Acc.) was obtained both by
<WT> (83.3%, Sens.; 55.6%, Spec.) and SD[WT]
(100.0%, Sens.; 44.4%, Spec.). MCI identification is
a crucial issue to establish an early AD diagnosis
(Morris, 2012). Classification rates about 65% have
usually been reported by previous MEG studies
(Bruña et al., 2012; Escudero et al., 2011; Fernández
et al., 2006, 2010; Gómez et al., 2009). Thus, it is
worth noting that our classification results are
comparable or even higher than those reported in
several MEG studies, though they did not use any
LOO-CV procedure (Fernández et al., 2006, 2010;
Figure 2: Spatial distribution of the parameters extracted from the WT and its significant difference between healthy
controls and MCI subjects. (a) <WT>; (b) SD[WT].
IJCCI2012-InternationalJointConferenceonComputationalIntelligence
724

Gómez et al., 2009). Certainly, the diagnostic
performance of the parameters could have been
evaluated using other classification strategies, such
as the division into independent training and test
sets. However, due to the limited size of the dataset,
a LOO-CV procedure might provide more general
results.
Table 1: Results of the ROC-LOO-CV analysis using the
averaged <WT> and SD[WT] for the significant channels.
Parameter
Sens.
(%)
Spec.
(%)
Acc.
(%)
AUC
<WT> 83.3 % 55.6 66.7 0.704
SD[WT] 100.0 44.4 66.7 0.698
Finally, a number of limitations merit further
consideration. In this study, the computation of the
WT was based on the CWT with a real Morlet
wavelet. However, other time-frequency
representations or other “mother” wavelets could
also be considered. Thus, the STFT has been
previously used to compute the so-called spectral
turbulence (ST), which is analogous to the WT. In
an early study, the ST was applied to characterize
MEG activity in AD (Poza et al., 2008b). In line
with our findings, their results support the notion
that a significant decrease in irregularity can be
observed in brain dynamics due to dementia
progression. Likewise, some researchers applied
complex-valued wavelets to describe EEG brain
dynamics (Vialatte et al., 2011). Further efforts
should be devoted to exploring the differences that
can arise in the characterization of MEG activity
using real- or complex-valued wavelets. It should be
appropriate to increase the cohort of subjects
enrolled in the study. Other neurodegenerative
disorders also exhibit similar abnormalities to those
reported in the present study. Further studies are
required to accurately characterize the specific
patterns associated to each disease. In addition, a
longitudinal analysis would be appropriate to
analyze the different patterns between MCI patients
that subsequently progress to AD and those who do
not develop AD.
5 CONCLUSIONS
In summary, our findings suggest that spontaneous
MEG activity in MCI is characterized by a
significant loss of irregularity, in terms of the degree
of similarity and variability in the spectral content.
The WT extend the concept of irregularity in
comparison to spectral and non-linear entropies.
Therefore, this parameter may lead to a better
understanding of the underlying brain dynamics in
MCI.
Future research will explore other definitions of
WT based on different “mother” wavelets and time-
frequency representations. Likewise, further efforts
will be addressed to analyze the WT patterns in
other dementias.
ACKNOWLEDGEMENTS
The authors would like to thank the “Asociación de
Familiares de Enfermos de Alzheimer” and the
Geriatric Unit of the “Hospital Clínico Universitario
San Carlos” for recruiting the subjects who
participated in this study.
This research was supported in part by: the
Ministerio de Economía y Competitividad and
FEDER under project TEC2011-22987; the
‘Proyecto Cero 2011 on Ageing’ from Fundación
General CSIC, Obra Social La Caixa and CSIC; and
project VA111A11-2 from Consejería de Educación
(Junta de Castilla y León).
REFERENCES
Albert, M. S., DeKosky, S. T., Dickson, D., Dubois, B.,
Feldman, H. H., Fox, N. C., Gamst, A., Holtzman, D.
M., Jagust, W. J., Petersen, R. C., Snyder, P. J.,
Carrillo, M. C., Thies, B., Phelps, C. H. (2011). The
Diagnosis of Mild Cognitive Impairment due to
Alzheimer’s Disease: Recommendations from the
National Institute on Aging-Alzheimer’s Association
Workgroups on Diagnostic Guidelines for Alzheimer’s
Disease. Alzheimer’s & Dementia: The Journal of the
Alzheimer’s Association, 7(3):270-279.
Baraniuk, R. G., Flandrin, P., Janssen, A. J. E. M. and
Michel, O. J. J. (2001). Measuring Time-Frequency
Information Content using the Rényi Entropies IEEE
Transactions on Information Theory, 47(4), 1391-
1409.
Barbosa, P. R. B., de Souza, A., Barbosa, E. C., Ginebra,
P., Cardoso, S. H., Destro, C. and Nadal, J. (2006).
Spectral Turbulence Analysis of the Signal-Averaged
Electrocardiogram of the the Atrial Activation as
Predictor of Recurrence of Idiopathic and Persistent
Atrial Fibrillation. International Journal of
Cardiology, 107(3), 307-316.
Blanco, S., García, H., Quiroga, R. Q., Romanelli, L. and
Rosso, O. A. (1995). Stationarity of the EEG series.
IEEE Engineering in Medicine and Biology Magazine,
14(4), 395-399.
Blennow, K., de Leon, M. J. and Zetterberg, H. (2006).
Alzheimer’s disease. Lancet, 368(9533), 387-403.
AnalysisofSpontaneousMEGActivityinMildCognitiveImpairmentusingtheWaveletTurbulence
725

Bruña, R., Poza, J., Gómez, C., Fernández, A., García, M.
and Hornero, R. (2012). Analysis of Spontaneous
MEG Activity in Mild Cognitive Impairment and
Alzheimer’s Disease using Spectral Entropies and
Statistical Complexity Measures. Journal of Neural
Engineering, 9(3), 036007.
Escudero, J., Sanei, S., Jarchi, D., Abásolo, D. and
Hornero, R. (2011). Regional Coherence Evaluation in
Mild Cognitive Impairment and Alzheimer’s Disease
based on Adaptively Extracted
Magnetoencephalogram Rhythms. Physiological
Measurement, 32(8), 1163-1180.
Fernández, A., Hornero, R., Mayo, A., Poza, J., Gil-
Gregorio, P. and Ortiz, T. (2006). MEG Spectral
Profile in Alzheimer’s Disease and Mild Cognitive
Impairment. Clinical Neurophysiology, 117(2), 306-
314.
Fernández, A., Hornero, R., Gómez, C., Turrero, A., Gil-
Gregorio, P., Matías-Santos, J. and Ortiz, T. (2010).
Complexity Analysis of Spontaneous Brain Activity in
Alzheimer’s Disease and Mild Cognitive Impairment:
an MEG Study. Alzheimer Disease and Associated
Disorders, 24(2) 182-189.
Figliola, A. and Serrano, E. (1997). Analysis of
Physiological Time Series using Wavelet Transforms.
IEEE Engineering in Medicine and Biology Magazine,
16(3), 74-79.
Gómez, C., Stam, C. J., Hornero, R., Fernández, A. and
Maestú, F. (2009). Disturbed Beta Band Functional
Connectivity in Patients with Mild Cognitive
Impairment: an MEG Study. IEEE Transactions on
Biomedical Engineering, 56(6), 1683-1690.
Kelen, G. J., Henkin, R., Starr, A. M., Caref, E. B.,
Bloomfield, D. and el-Sherif, N. (1991). Spectral
Turbulence Analysis of the Signal-Averaged
Electrocardiogram and its Predictive Accuracy for
Inducible Sustained Monomorphic Ventricular
Tachycardia. American Journal of Cardiology, 67(11),
965-975.
Morris, J. C. (2012). Revised Criteria for Mild Cognitive
Impairment May Compromise the Diagnosis of
Alzheimer Disease Dementia. Achives of Neurology,
69(6), 700-708.
Percival, D. B. (1995). On Estimation of the Wavelet
Variance. Biometrika, 82(3), 619-631.
Petersen, R. C., Doody, R., Kurz, A., Mohs, R. C., Morris,
J. C., Rabins, P. V., Ritchie, K., Rossor, M., Thal, L.
and Winblad, B. (2001). Current Concepts in Mild
Cognitive Impairment. Archives of Neurology, 58(12),
1985-1992.
Petersen, R. C. and Morris, J. C. (2003). Conceptual
Overview. In Petersen, R. C. (Ed.), Mild Cognitive
Impairment: Aging to Alzheimer’s Disease (pp. 1-14).
New York: Oxford University Press.
Poza, J., Escudero, J., Hornero, R., Fernández, A. and
Sanchez, C. I. (2008a). Regional Analysis of
Spontaneous MEG Rhythms in Patients with
Alzheimer’s Disease using Spectral Entropies. Annals
of Biomedical Engineering, 36(1), 141-152.
Poza, J., Hornero, R., Escudero, J., Fernández, A. and
Gómez, C. (2008b). Analysis of Spontaneous MEG
Activity in Alzheimer’s Disease using Time-
Frequency Parameters. In IEEE-EMBC’08 30th
Annual International Conference of the IEEE
Engineering in Medicine and Biology Society, pp.
5712-5715.
Rampp, S. and Stefan, H. (2007). On the Opposition of
EEG and MEG. Clinical Neurophysiology, 118(8),
1658-1659.
Reitz, C., Brayne, C. and Mayeux, R. (2011).
Epidemiology of Alzheimer Disease. Nature Reviews.
Neurology, 7(3), 137-52.
Stam, C. J. (2010). Use of Magnetoencephalography
(MEG) to Study Functional Brain Networks in
Neurodegenerative Disorders. Journal of the
Neurological Sciences, 289(1-2), 128–134.
Vialatte, F. B., Dauwels, J., Maurice, M., Musha, T. and
Cichocki, A. (2011) Improving the Specificity of EEG
for diagnosing Alzheimer’s Disease. International
Journal of Alzheimer’s Disease, 2011, 259069.
IJCCI2012-InternationalJointConferenceonComputationalIntelligence
726
