
CyberBrain
A Preliminary Experience on Non Human Primate
M. Piangerelli
1
, A. Paris
2
and P. Romanelli
2
1
Computer Science Division, University of Camerino, Via del Bastione 1, 62032, Camerino, Italy
2
AB Medica s.p.a., via Nerviano 31, 20020, Lainate, Milano, Italy
Keywords:
Epilespy, Wireless EcoG device, Closed-loop Device, Abnormal Brain Activity.
Abstract:
The study of abnormal electrical activity of the brain, such as epilepsy, is attracting more and more interest for
its wide impact on the population. Intracranial EEG recording (electrocorticogaphy; EcoG) and direct cortical
stimulation (DCS) are, nowadays, the most accurate and reliable techniques to map cortical function and to
identify the boundaries of an epileptic focus. In this work we present the preliminary testing of intra-operative
ECoG and DCS performed in a non-human primate using a new custom-made fully-implantable wireless 16-
channels device (Patent Number: WO2012143850), called ECOGW-16E. This fully-integrated device, housed
in a compact hermetically sealed Polyetheretherketone (PEEK) enclosure, exploits the newly available Med-
ical Implant Communication Service band (MICS: 402-405 MHz). ECOGW-16E is wirelessly rechargeable
using a special designed cage for recharge, developed in accordance with guidelines for accommodation of
animals by Council of Europe.
1 INTRODUCTION
Multichannel electrophysiological measurements of
neural activity are a fundamental method in neu-
ral research and in medical applications. Nowa-
days Intracranial EEG recording (electrocorticogra-
phy, EcoG) and direct cortical stimulation (DCS) are
the gold standard techniques to map the cortical func-
tions and to identify the epileptogenic foci (Spena
et al., 2010; Borchers et al., 2012) but provide also an
exquisite tool to assess the function and interactions
of neuronal ensembles. For example, observing the
activity in a great number of simultaneously measured
channels is necessary for understanding the functions
of the visual cortex, where the signals from many mil-
lions of neurons represent the first stages of creating
our visual perceptions. Electrode arrays are essential
tools to localize the source of epileptic seizures and
later remove these parts from the brain during a sur-
gical intervention (Crone et al., 1998): the higher the
spatial resolution of the array, the less healthy tissue
needs to be removed from the brain. Finally in a not
so distant future, integrated devices provided with ap-
propriate algorithms, can deliver performant closed-
loop stimulation modulating brain function. Closed-
loop stimulation can be used to abort seizures or to
provide countless potential intervention opportunities
to counteract brain disorders such as pain, tremor and
Parkinsons disease (Warren and Durand, 1998; Moro
et al., 2002; Johnson et al., 2013). Todays elec-
trode arrays are mostly connected via cables through
the skull which always brings the risk of infections,
limiting the observation window to short-term only.
There is therefore a strong need to develop wireless
systems able to extend substantially the monitoring
window, up to a chronic use. Our group developed
a highly innovative wireless externally-rechargeable
multi-channel EcoG device. Preliminary results of ex-
perimental tests on a monkey will be presented here.
2 MATERIALS & METHODS
2.1 Surgical Process
One male macaque monkey was used in this study
(Macaca fascicularis, 6.95 kg). Experimental proto-
col was approved by the regional committee (Cometh
Grenoble) and registered to the national commit-
tee under the number 12/136
Clinatec-NTM-01 and
complied with the EU directive 22th September 2010
(2010/63/EU ) on the care and use of laboratory an-
imals. MRI was performed prior to surgical explo-
94
Piangerelli M., Paris A. and Romanelli P..
CyberBrain - A Preliminary Experience on Non Human Primate.
DOI: 10.5220/0005089700940098
In Proceedings of the 2nd International Congress on Neurotechnology, Electronics and Informatics (NEUROTECHNIX-2014), pages 94-98
ISBN: 978-989-758-056-7
Copyright
c
2014 SCITEPRESS (Science and Technology Publications, Lda.)
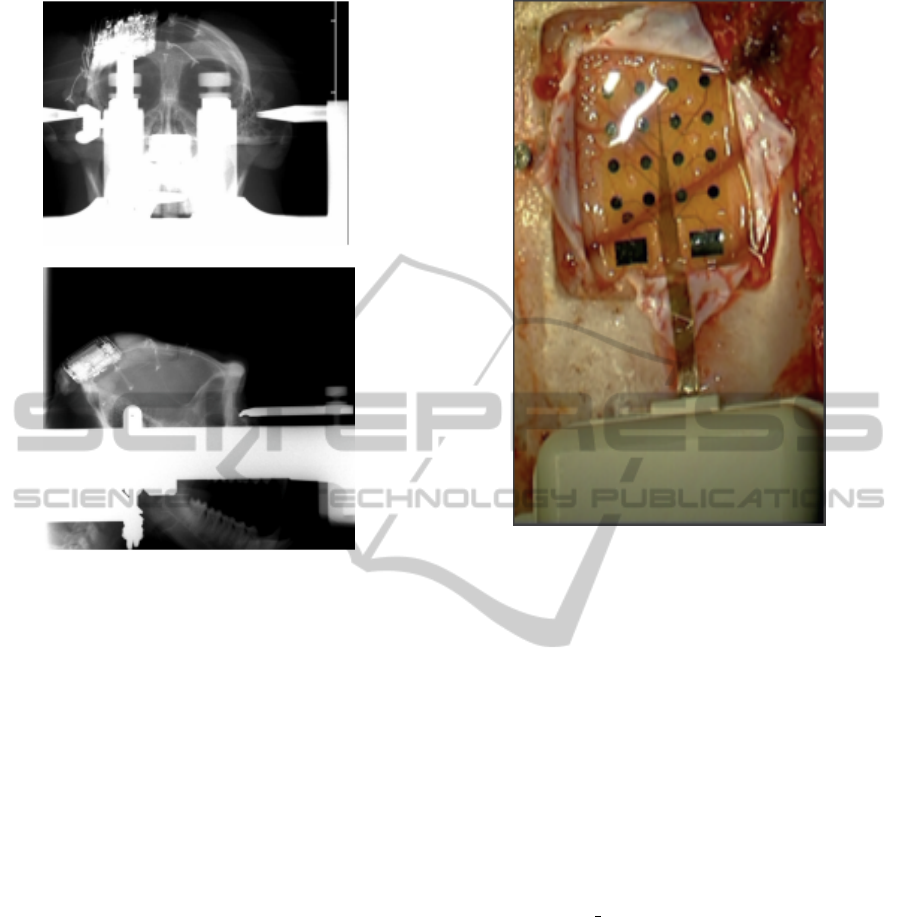
Figure 1: Post-op radiographic image of the device.
ration to define surgical management. The animal was
anesthetized using a loading dose Xylazine 5 mg/kg,
and Ketamine hydrochloride 20 mg/kg, IM and then
a maintenance dose of 1.25 mg/kg, 5mg/kg Xylazine
/Ketamine respectively. Heart rate, blood pressure,
respiratory depth, and body temperature were con-
stantly monitored by veterinary staff. Surgical proce-
dures took place in standard aseptic conditions. When
deep anesthesia was achieved, the animal was secured
to a stereotaxic frame, and a craniotomy was per-
formed over the left motor cortex (M1), in Brodmann
area 4. The dura mater was cut in a Y fashion and
the leafs were retracted and sutured on the sides in
order to expose the central sulcus and the surfaces
of the primary motor(M1) and sensory (S1) cortex.
Radiographic images (Fig.1) during surgery were ac-
quired to guide device placement. The device was po-
sitioned orthogonally and the grid was centered above
the hand knob of the left motor cortex (Fig.2).
2.2 ECOGW-16E
The device, called ECOG-16W (Cristiani et al., 2012)
(total length 58.3 mm), specifically designed for mon-
keys, consits of two parts (Fig.3 ): the grid (Fig.4),
consisting of a single sheet of flexible polyimide
support that integrates 16 platinum electrodes (Neu-
ronexus, Ann Arbor, MI, USA) and the body, cas-
Figure 2: Device placement over the sensorimotor cortex:
the central sulcus is visible under the grid
ing in PEEK, includes a microcontroller handling lo-
cal processing and the transceiver module for im-
plantable medical applications within MICS band
with a 800/400/200 kbps raw data rate. In addition,
it includes a triaxial accelerometer, a stimulus gener-
ator, a sensor of temperature/load current and a Li-
Ion battery(3,6V 150mA/h ISO 13485). PEEK case
was chosen for its high and well documented biocom-
patibilty in prosthesis in different field of medicine
(Rivard et al., 2002). Finally, we have adopted a
charging apparatus to provide an induction charger
(250mW, 70mA @ 3.7V) for a wireless recharge-
able battery. The interface consumes 58mA(16CH
@ 500SPS + TX RF), 30mA(16CH @ 500SPS) and
7mA in standby. The device was positioned orthogo-
nally and the grid was centered above the hand knob
of the left motor cortex (Fig.2) providing also cover-
age of the central sulcus and part of S1.
ECOGIW-16E is wirelessly rechargeable using a
special designed cage for recharge (Fig. 5).
Wired systems expose the cables to the risk of
damage induced by the monkey , which pulls them
away. To perform behavioral experiments, the mon-
key needs to seat in a special chair that does not allow
the arms of the animal to reach the cables. Our cable
free system opens therefore a new window of oppor-
tunities to observe the neural activities in unrestrained
animals. Using the smart wireless recharge cage it is
CyberBrain-APreliminaryExperienceonNonHumanPrimate
95
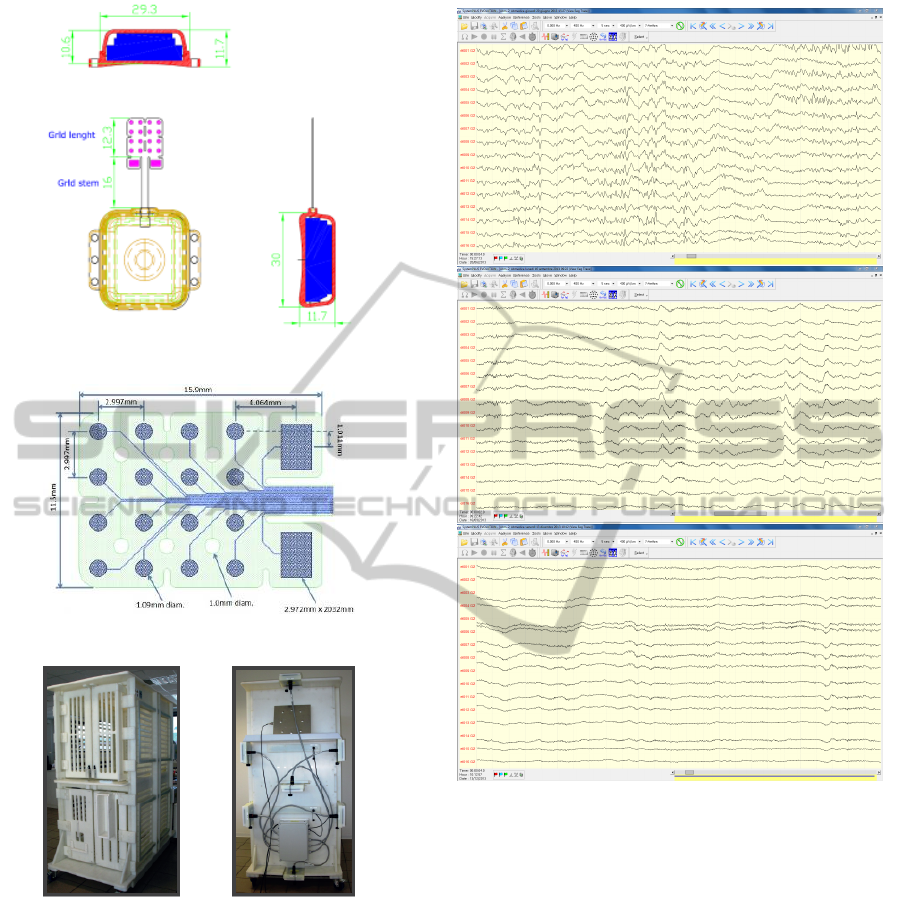
Figure 3: The scheme of the ECOG-16E.
Figure 4: The grid by NeuroNexus.
Figure 5: The recharge cage.
also possible to recharge the implanted device during
the physiological rest period.
3 DATA ANALYSIS AND RESULTS
In this study we used a commercial software for
ECoG recording (Micromed, Treviso; Italy) and we
tested software specifically developed for our hard-
ware (Aethra Communication company; Ancona,
Italy) for DCS and impedance measurement. The
procedure and post-operatory course was uneventful.
The monkey recovered immediately and was able to
Figure 6: Tipical Ecog traces: after the implant (top), after
three months (middle) and after six months (bottom).
resume all the normal motor activities within a few
hours. The ECoG signals of sixteen electrodes was
recorded with a 512 Hz sampling rate and a software-
imposed band-pass filter from 0.008-400 Hz. We first
removed all frequencies below 0.5 Hz from the ECoG
signals using a high-pass filter. EcoG signal recording
was performed every day for 6 months and remained
excellent for all the time (Fig.6). In the figure 6 three
screenshots are shown: after few hours since the im-
plant, after three months and after six months. It is
possible to notice the high fidelity of the signal with
no visible distortion and noise.
We measured, every day, the values of impedance
at different frequencies (from 16Hz to 2048Hz) in
all channel (Fig.7); in our experiment, impedance is
measured at different frequencies; as you can see in
figure 7, the values of the impedance for each elec-
NEUROTECHNIX2014-InternationalCongressonNeurotechnology,ElectronicsandInformatics
96
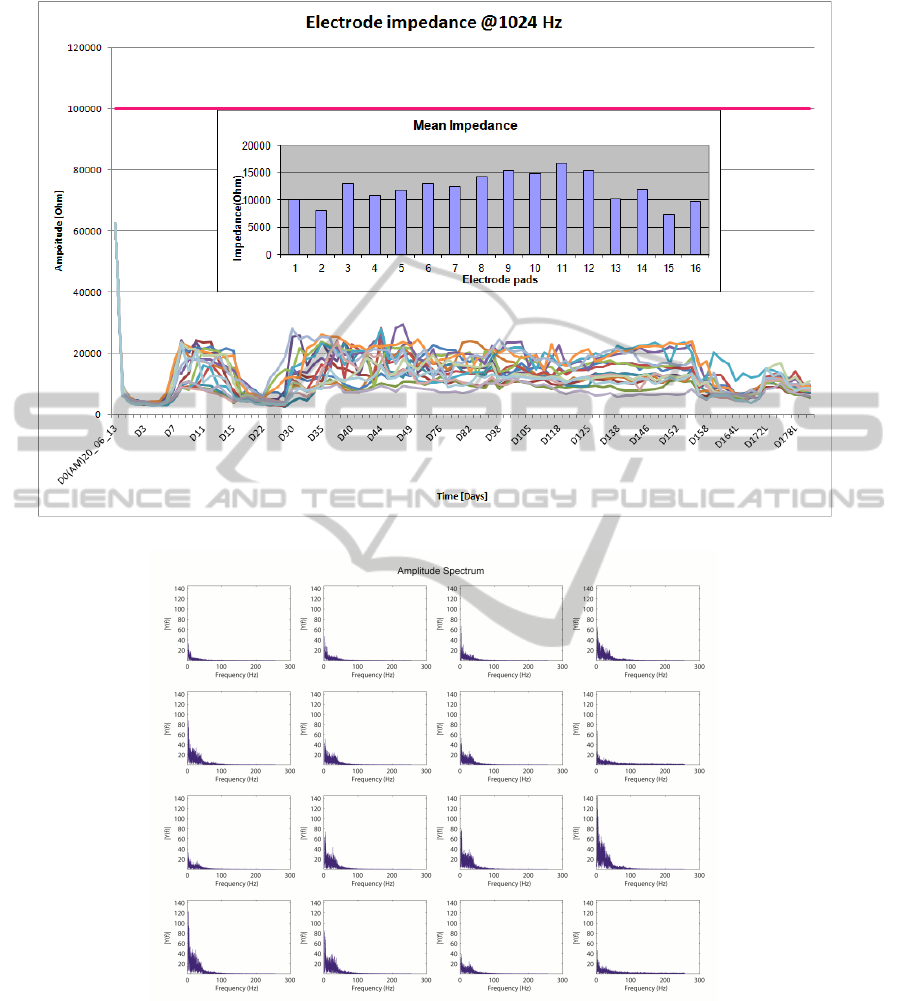
Figure 7: Impedance values over time
Figure 8: Impedance values over time.
trode is got @1kHz; in the inset the mean value for
each electrode is also reported. For all the days the
device has been implanted, impedance values has re-
mained under the horizontal pink line (@100 KHz),
representing the maximum limit of the impadance.
Our device has impedance values consistent to the
ones found in literature (Kellis et al., 2011). We
computer, also, the Fast Fourier Transform (FFT) of
the ECoG traces. The frequency spectrum shows
the characteristic decrease in amplitude at higher fre-
quencies and also that there are all the characteris-
tic frequency components of an ECoG signal (Fig.8).
Cortical stimulation was performed with a protocol
planning to stimulate the primate every seven days.
We performed bipolar stimulation by pulses of rectan-
gular shapes with anodal monophasic current pulses
CyberBrain-APreliminaryExperienceonNonHumanPrimate
97

of 0.5 ms duration. The stimulation technique con-
sists of a train of 5 pulses delivered at 1 Hz, which
is equivalent to an interstimulus interval of 100 ms.
Stimulus intensity was gradually increased in incre-
ments of 0.5 mA, starting at 1 mA, up to a maximum
of 3 mA. We testing all the contact with a reference
electrode positioned in the left of grid. During corti-
cal stimulation of the expected motor cortex, move-
ments of distinct portions of the right arm were ob-
served with a stimulation intensity of 2 mA.
4 CONCLUSIONS
The implantable wireless device, here presented, al-
low us to perform chronic EcoG recording and DCS.
research and clinical applications of this novel tech-
nology include brain mapping, seizure foci localiza-
tion and BCI. Wireless technology for transmitting
EcoG signal provides several advantages: risk and
limitation related to the presence of subcutaneous
connecting cables (Behrens et al., 1997) are removed
and the recording time can be substantially prolonged.
This fully-integrated system can also be used as a
closed loop system providing electrical stimulation
“on-demand” to abort promptly detected seizure ac-
tivity in drug-resistant epilepsy patients.
REFERENCES
Behrens, E., Schramm, J., Zentner, J., and K
¨
onig, R. (1997).
Surgical and neurological complications in a series
of 708 epilepsy surgery procedures. Neurosurgery,
41(1):1–10.
Borchers, S., Himmelbach M, Logothetis, N., and Karnath,
H. (2012). Direct electrical stimulation of human cor-
tex: the gold standard for mapping brain functions?
Nature Reviews Neuroscience, 13:63–70.
Cristiani, P., Marchetti, S., Paris, A., Romanelli, P., and
Sebastiano, F. (2012). Implantable device for ac-
quisition and monitoring of brain bioelectric signals
and for intracranial stimulation. WO Patent App.
PCT/IB2012/051,909.
Crone, N. E., Miglioretti, D. L., Gordon, B., and Lesser,
R. P. (1998). Functional mapping of human sensori-
motor cortex with electrocorticographic spectral anal-
ysis. ii. event-related synchronization in the gamma
band. Brain, 121(12):2301–2315.
Johnson, L. A., Wander, J. D., Sarma, D., Su, D. K., Fetz,
E. E., and Ojemann, J. G. (2013). Direct electrical
stimulation of the somatosensory cortex in humans us-
ing electrocorticography electrodes: a qualitative and
quantitative report. Journal of Neural Engineering,
10(3):036021.
Kellis, S., Greger, B., Hanrahan, S., House, P., and Brown,
R. (2011). Platinum microwire for subdural elec-
trocorticography over human neocortex: Millimeter-
scale spatiotemporal dynamics. In Engineering in
Medicine and Biology Society,EMBC, 2011 Annual
International Conference of the IEEE, pages 4761–
4765.
Moro, E., Esselink, R. J., Xie, J., Hommel, M., Benabid,
A. L., and Pollak, P. (2002). The impact on parkinsons
disease of electrical parameter settings in stn stimula-
tion. Neurology, 59(5):706–713.
Rivard, C.-H., Rhalmi, S., and Coillard, C. (2002). In
vivo biocompatibility testing of peek polymer for a
spinal implant system: A study in rabbits. Journal
of Biomedical Materials Research, 62(4):488–498.
Spena, G., Nava, A., Cassini, F., Pepoli, A., Bruno, M.,
DAgata, F., Cauda, F., Sacco, K., Duca, S., Barletta,
L., et al. (2010). Preoperative and intraoperative brain
mapping for the resection of eloquent-area tumors. a
prospective analysis of methodology, correlation, and
usefulness based on clinical outcomes. Acta neu-
rochirurgica, 152(11):1835–1846.
Warren, R. and Durand, D. M. (1998). Effects of applied
currents on spontaneous epileptiform activity induced
by low calcium in the rat hippocampus. Brain Re-
search, 806(2):186 – 195.
NEUROTECHNIX2014-InternationalCongressonNeurotechnology,ElectronicsandInformatics
98
