
Dielectrophoretic Characteristics of Microbeads Labeled with DNA
of Various Lengths
Zhenhao Ding
1
, Hiromichi Kasahara
1
, Michihiko Nakano
2
and Junya Suehiro
2
1
Graduate School of Information Science and Electrical Engineering, Kyushu University, 744 Motooka, Fukuoka, Japan
2
Faculty of Information Science and Electrical Engineering, Kyushu University, 744 Motooka, Fukuoka, Japan
Keywords: DNA Labelling, Dielectrophoresis, Rapid DNA Detection, Crossover Frequency.
Abstract: Polymerase chain reaction (PCR) is one of the most sensitive and specific detection methods of bacterial
and viral infections. The authors proposed a new electrical technique for rapid detection of DNA amplified
by PCR using dielectrophoresis (DEP) of microbeads. The method is based on dramatic alteration of DEP
characteristics of microbeads caused by DNA labelling. DNA labeled microbeads are trapped on a
microelectrode under the action of positive DEP, whereas pristine microbeads are not. DEP-trapped
microbeads can be measured impedimetrically to realize rapid and quantitative detection of the amplified
DNA. In this study, it was aimed to reveal how DNA length affects DEP characteristic of DNA-labeled
microbeads. Dielectrophoretic crossover from the negative to the positive was measured for microbeads
labeled with DNA length in 204 bp, 391 bp and 796 bp. After theoretical fitting of DEP crossover data, it
was revealed that the surface conductance increased when the length of labeled DNA increased.
1 INTRODUCTION
There are several methods to diagnose bacterial or
viral infections in human and animals. A nucleic
acid amplification test (NAT) is a highly sensitive
and specific method for detecting DNA or RNA of a
target pathogen. Polymerase chain reaction (PCR) is
a type of NAT used to amplify specific regions of
DNA or RNA via enzymatic reaction. PCR is a
widespread application in several areas of genetic
analysis (Storch, 2000; Malorny, 2003; Chung et al.,
2006).
DNAs amplified by PCR, amplicons, are
generally separated by size and detected by agarose
gel electrophoresis. Although this method is well
established and reliable, it requires rather
complicated and time-consuming manual operations
by experts. To overcome this drawback, real-time
PCR has emerged as an improvement for rapid
analysis. Real-time PCR optically detects amplicons
during PCR using a fluorescent probes that bind to
DNA. The fluorescence intensity increases with the
number of amplicons during the amplification.
However, the apparatus for real-time PCR is
expensive, almost 10 times the cost of a general
PCR equipment. Moreover, special knowledge and
experience are required to design fluorescent DNA
probes for optical detection (Mackay, 2002). Hence,
rapid, simple and economical amplicons detection
method was required.
The authors develop and demonstrate a novel
electrical method for detection of amplicons by
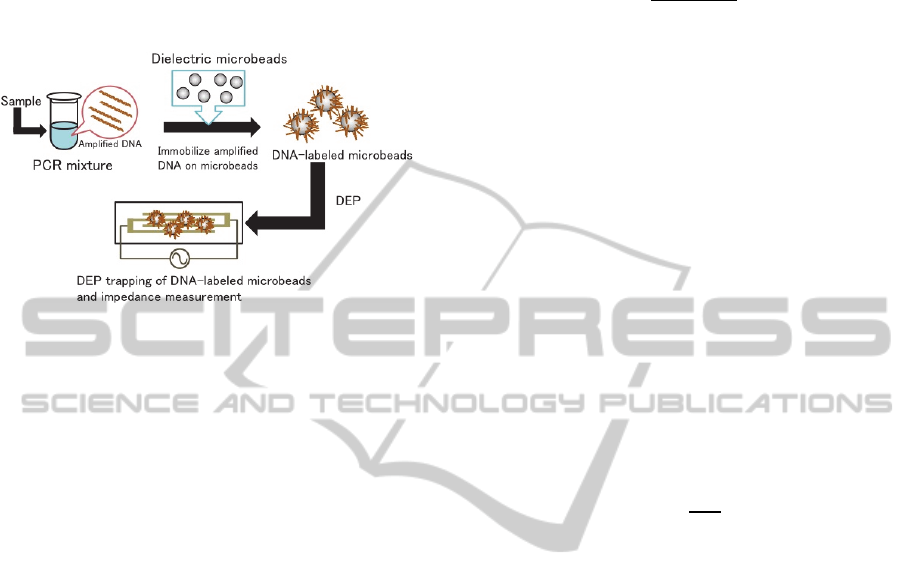
dielectrophoresis (DEP) of microbeads (Nakano et
al., 2014). In the method (Figure 1), the amplicons
are chemically immobilized on dielectric microbeads
so that DNA immobilization alters the DEP
characteristics of the microbeads. DNA-labeled
microbeads are trapped on a microelectrode under
the action of positive DEP, whereas pristine ones are
not trapped. Combining this dramatic alteration in
DEP phenomena with impedance measurement
allows rapid and quantitative detection of the
amplicons. An electrical detection technique called
dielectrophoretic impedance measurement (DEPIM),
which was originally developed by the authors’
group for bacterial and viral inspection, can be used
for the impedance measurement (Suehiro et al.,
1999). It was demonstrated that DNA-labeled
microbeads were trapped in the electrode gap, which
caused a detectable change in the electrode
impedance in a few seconds, whereas pristine
microbeads would repelled from the electrode gap,
which resulted in no impedance change (Nakano et
185
Ding Z., Kasahara H., Nakano M. and Suehiro J..
Dielectrophoretic Characteristics of Microbeads Labeled with DNA of Various Lengths.
DOI: 10.5220/0005280701850189
In Proceedings of the International Conference on Biomedical Electronics and Devices (BIODEVICES-2015), pages 185-189
ISBN: 978-989-758-071-0
Copyright
c
2015 SCITEPRESS (Science and Technology Publications, Lda.)
al., 2014). Hence, this method would provide rapid
detection of DNAs amplified by PCR, which may be
applicable to rapid, quantitative, and automated
diagnosis of bacterial and viral infections. However,
this method still required separation and selective
detection of PCR amplified DNA of different length.
Figure 1: Schematic illustration of the microbeads-based
detection of amplicons. After PCR amplification,
amplicons (amplified DNA) are chemically immobilized
on the microbeads. The DNA-labeled microbeads behaves
positive DEP, whereas the pristine microbeads behaves
negative DEP. The DEP-trapped microbeads can be
detected by DEPIM
This study aim to reveal how the length of
labelling DNA affects DEP characteristics of DNA
labeled microbeads. Alteration of DNA-labeled
microbeads DEP characteristics, which is affected
by the length of labeled DNA, can lead to rapid
separation and selective detection of PCR amplified
DNA of different length by impedance
measurement. Crossover frequency of the DEP for
microbeads labeled with DNA of different length
were measured at different suspending medium
conductivities. The alteration of DEP charateristics
was analysed using theoritical fitting of measured
data.
2 THEORY
DEP is the electrokinetic motion of dielectrically
polarized materials in non-uniform electric fields,
and it is currently an active area of research for
manipulation of biological particles and
nanomaterials, including bacterial cells and DNA
molecules (Pethig, 2010, Hughes, 2000, Ausbury et
al., 2002,). The DEP force acting on a spherical
dielectric particle of radius r suspended in a medium
of absolute permittivity ε
m
is given by as follows
2
Re
(1)
where E is the magnitude of the applied field.
Re[K(ω)] is the real component of the Clausius–
Mossotti (CM) factor, given by
∗
∗
∗
2
∗
(2)
where ε*
p
and ε*
m
are the complex permittivities of
the particle and the surrounding medium,
respectively. For a real dielectric, the complex
permittivity is defined as ε
*
ε‐j
σω
⁄
, where ε is
the permittivity, σ is the conductivity of the
dielectric, and ω is the angular frequency of the
applied electric field. When Re[K(ω)] has a positive
value, the particle is propelled toward the high field
region (positive DEP, p-DEP). With a negative value
of Re[K(ω)], the particle is repelled from the high
field region (negative DEP, n-DEP). The crossover
frequency f
x
is defined as the value of the applied
frequency which results in the cessation of particle
motion. Therefore, measurement of the crossover
frequency can used to characterize the dielectric
properties of single particle.
The conductivity of a solid dielectric particle, σ
p
,
can be expressed by the following equation
(Ermolina and Morgan, 2005).
2
(3)
where σ
b
and K
s
are the bulk conductivity and the
surface conductance of the particle. Equations 1–3
imply that the dielectric properties and the resultant
DEP force acting on a smaller particle should be
more dependent on the surface conductance K
s
.
Hughes et al. reported that antibody (protein)
coating of submicrometer latex spheres altered the
surface conductance and DEP spectrum of the
particles, enabling the separation of unlabeled and
protein-labeled particles (Hughes and Morgan,
1999). Zhou et al. found that the dielectric properties
of microbeads were modified by coating with
bacterial biofilms, resulting in an altered
electrorotation spectrum (Zhou et al., 1995).
3 EXPERIMENTS
We used pUC 19 DNA as template for PCR. The 5’
end of forward primers, which were designed for
amplifications of 204 bp DNA, 391 bp DNA and
796 bp DNA from pUC 19, were tagged with biotin.
As the results of PCR, 204 bp DNA, 391 bp DNA
and 796 bp DNA were amplified and these
amplicons were confirmed by standard agarose gel
electrophoresis.
BIODEVICES2015-InternationalConferenceonBiomedicalElectronicsandDevices
186

Magnetic microbeads (Dynabeads
®
M-280, Life
Technologies 2.8 μm in diameter) were used in this
experiment. The surface of the microbeads is coated
with streptavidin, which binds specifically to biotin.
Microbeads (3x10
4
beads/μl) were mixed with the
reaction solution (5 mM Tris-HCl (pH 7.5), 0.5 mM
EDTA, 1 M NaCl), including the amplicons of 204
bp, 391 bp and 796 bp separately. The amplicon
concentrations in the solution were approximately
3.5x10
10
DNA/μl. The mixtures of the amplicons
and microbeads were incubated at room temperature
for 15 minutes. Hence, the microbeads were
functionalized with amplicons via biotin-streptavidin
interaction. Then, the DNA labeled microbeads were
suspended in deionized water (conductivity 2 × 10
−4
S/m).
A castle-walled microelectrode having the
narrowest gap of 5 μm, which is shown as black part
in Figure 2, was used. DEP behaviors of microbeads
labeled with DNA of different length were observed
with an inverted microscope equipped with a CCD
camera. The DNA-labeled microbeads were
suspended in NaCl solution of concentration range
from 5 μM to 1 mM. Then, 5μl of the solution
containing the DNA-labeled microbeads was placed
on the microelectrode and covered with a cover slip.
An AC voltage of 20 V
peak-to-peak
was applied to the
microelectrode to generate DEP force. The DEP
crossover frequency was measured by observing
DNA-labeled microbeads motion with varying
applied voltage frequency. The DEP force changed
from negative DEP, where microbeads were repelled
from the electrode gap, which is the high electric
field (Figure 2. a.) to positive DEP, where
microbeads were trapped in the electrode gap
(Figure 2. b.) along with the decreasing of applied
voltage frequency. The DEP crossover frequency
was determined as the frequency when the DEP
force changed from n-DEP to zero (Figure 2. c.).
4 RESULTS AND DISCUSSION
The crossover data for microbeads labeld by DNA
of 204 bp, 391 bp and 796 bp in length are shown in
Figure 3. The data are plotted for DNA-labeled
microbeads suspended in NaCl solution of different
conductivities range from 10
-4
to 10
-1
S/m. At high
suspending medium conductivities the DNA labeled
microbeads experienced only negative DEP. At
suspending medium conductivities below 10
-3
S/m,
the crossover frequency was clearly dependent on
the length of labeled DNA. For example, Figure 4
shows that at suspending medium conductivity of
2x10
-4
S/m, the crossover frequency became higher
when the length of labeled DNA increased. This
suggests that applying voltage of appropriate
frequency can separate microbeads labeled by DNA
of different length. For example, as shown in Figure
3, in suspending medium conductivity of 2x10
-4
S/m, if the frequency between 3.1 x 10
6
Hz and 3.8 x
10
6
Hz is applied, 796 bp-DNA-labeled microbeads
would experience positive DEP, while 391 bp-DNA-
labeled microbeads and 204 bp-DNA-labeled
microbeads would experience negative DEP.
a. Negative DEP
b. Positive DEP
c. No DEP force
Figure 2: Optical images showing DEP behaviors of
DNA-labeled microbeads.
The solid lines in Figure 3 are the best fit to
model described by Equations 1 - 3 and the fitting
data are summarised in Table 1.
DielectrophoreticCharacteristicsofMicrobeadsLabeledwithDNAofVariousLengths
187
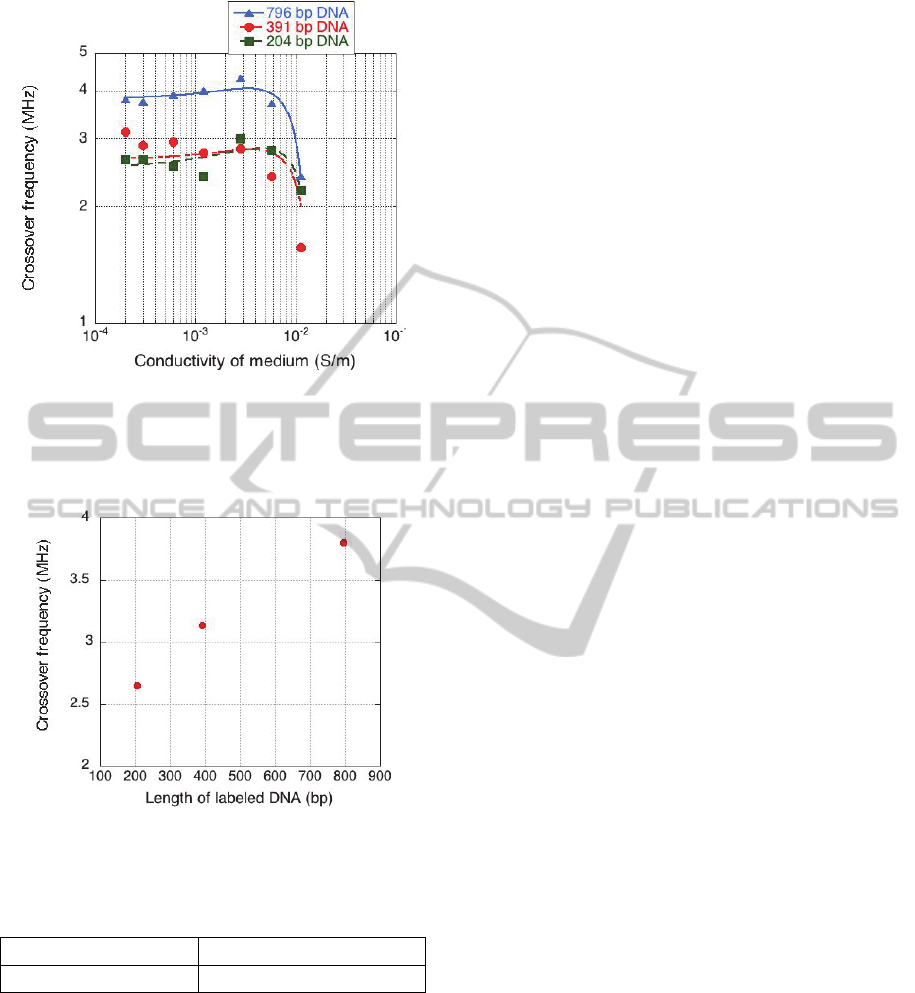
Figure 3: Crossover frequency for microbeads labeled by
DNA length in 204 bp, 391 bp and 796 bp plotted as a
function of suspending medium conductivity. Solid lines
are the best fir to the model described by Equation 1- 3.
The fitting data are summarized in Table 1.
Figure 4: Crossover frequency plotted as a function of
labeled DNA length.
Table 1: Fitting data of surface conductance of microbeads
labeled by DNA length in 204 bp, 391 bp and 796 bp.
Labeled DNA length (bp) 204 391 796
Surface conductance (nS) 9.45 10.29 11.28
Table 1 shows that the surface conductance of
DNA labeled microbeads increased with the DNA
length. This was because of the negative electrical
charges of DNA. Longer DNA has lager negative
electrical charges, therefore, the surface conductance
of DNA labeled microbeads will increase when the
length of labeled DNA increases, which will cause
the alteration of crossover frequency of DNA-
labeled microbeads.
5 CONCLUSIONS
Dielectrophoretic crossover frequency of
microbeads labeled by DNA of virous lengths were
measured at different suspending medium
conductivities. At suspending medium conductivities
below 10
-3
S/m, the crossover frequency was clearly
dependent on the length of labeled DNA that the
crossover frequency became higher when the length
of labeled DNA increased. After theoretical fitting
of DEP crossover data, it was revealed that the
surface conductance increased when the length of
labeled DNA increased. Hence, DEP characteristics
of DNA-labeled microbeads altered with DNA
length. Therefore, if voltage of appropriate
frequency were applied, longer DNA would
experience positive DEP while shorter DNA would
experience negative DEP, which could lead to rapid
separation and selective detection of PCR amplified
DNA of different length by impedance
measurement. Hence the proposed microbead-based
assay may provide rapid detection of DNAs
amplified from multiplex PCR, which may be
applicable to rapid, quantitative, and automated
diagnosis of bacterial and viral infections.
ACKNOWLEDGEMENTS
This work was partly supported by JSPS KAKENHI
Grant numbers 25820174, and 26289125.
REFERENCES
Ausbury, Charles L., Diercks, Alan, H., van den Engh,
Ger, 2002, Trapping of DNA by dielectrophoreis. In
Electrophoresis.
Chung, N. L., Nicholas, M. T., Richard, A. M., 2006,
Mutichannel PCR-CE Microdevice for Genetic
Analysis. In Analytical Chemictry.
Ermolina, I., Morgan, H., 2005. The electrokinetic
properties of latex particles: comparison of
electrophoresis and dielectrophoresis. In Journal of
Colloid and Interface Science.
Hughes, M. P., Morgan, H., 1999. Dielectrophoretic
manipulation and separation of surface-modified latex
microspheres. In Analytical Chemistry.
Hughes, M. P., 2000. AC electrokinetics: applications for
nanotechnology. In Nanotechnology.
Mackay, I., 2002. Real-time PCR in virology. In Nucleic
Acids Research.
Malorny, B., 2003. Standardization of diagnostic PCR for
the detection of foodborne pathogens. In International
Journal of Food Microbiology.
BIODEVICES2015-InternationalConferenceonBiomedicalElectronicsandDevices
188

Nakano, M., Ding, Z., Obara. R., Kasahara, H., Suehiro,
J., 2014. Rapid DNA detection based on direction
reversing of dielectrophoresis of DNA-attached
microbeads. In Biosensor 2014.
Pethig, R., 2010. Review article-dielectrophoresis: status
of the theory, technology and applications. In
Biomicrofluidics.
Storch, G. A., 2000. Diagnostic virology. In Clinical
Infectious Disease.
Suehiro, J., Yatsunami, R., Hamada, R. Hara, M., 1999.
Quanitative estimation of biological cell concentration
suspended in aqueous medium by using
dielectrophoretic impedance measurement method. In
Journal of Physics D: Applied Physics.
Zhou, X. F., Markx, G. H., Pethig, R., Eastwood, I. M.,
1996. Effect of biocide concentration on
electrorotation spectra of yeast cells. In Biochimica et
Biophysica Acta.
DielectrophoreticCharacteristicsofMicrobeadsLabeledwithDNAofVariousLengths
189
