
Towards a Unified Named Entity Recognition System
Disease Mention Identification
Tsendsuren Munkhdalai, Meijing Li, Khuyagbaatar Batsuren and Keun Ho Ryu
Database/Bioinformatics Laboratory, School of Electrical & Computer Engineering, Chungbuk National University,
Cheongju, South Korea
Keywords: Feature Learning, Semi-Supervised Learning, Named Entity Recognition, Conditional Random Fields.
Abstract: Named Entity Recognition (NER) is an essential prerequisite task before effective text mining can begin for
biomedical text data. Exploiting unlabeled text data to leverage system performance has been an active and
challenging research topic in text mining due to the recent growth in the amount of biomedical literature. In
this study, we take a step towards a unified NER system in biomedical, chemical and medical domain. We
evaluate word representation features automatically learnt by a large unlabeled corpus for disease NER. The
word representation features include brown cluster labels and Word Vector Classes (WVC) built by apply-
ing k-means clustering to continuous valued word vectors of Neural Language Model (NLM). The experi-
mental evaluation using Arizona Disease Corpus (AZDC) showed that these word representation features
boost system performance significantly as a manually tuned domain dictionary does. BANNER-
CHEMDNER, a chemical and biomedical NER system has been extended with a disease mention recogni-
tion model that achieves a 77.84% F-measure on AZDC when evaluating with 10-fold cross validation
method. BANNER-CHEMDNER is freely available at: https://bitbucket.org/tsendeemts/banner-chemdner.
1 INTRODUCTION
One essential task in developing an information
extraction system is the Named Entity Recognition
(NER) process, which basically defines the bounda-
ries between typical words and biomedical terminol-
ogy in a particular text, and assigns the terminology
to specific categories based on domain knowledge.
Gene and protein mention recognition in biomed-
ical text has been a main focus of the bio-text min-
ing community and many systems have been devel-
oped (Leaman 2008, Munkhdalai 2013). In contrast,
recognition of disease has received much less atten-
tion. Proposed solutions include rule-based, diction-
ary-based, and Machine Learning (ML) approaches.
In the dictionary-based approach, a prepared
terminology list is matched through a given text to
retrieve chunks containing the location of the termi-
nology words (Karopka 2006, Jimeno 2008, Gu-
rulingappa 2010). However, medical and chemical
text can contain new terminology that has yet to be
included in the dictionary.
The rule-based approach defines particular rules
by observing the general features of the entities in a
text. In order to identify any named entity in text
data, a rule-generation process has to process a huge
amount of text to collect accurate rules. In addition,
the rules are usually collected by domain experts,
requiring a lot of effort.
Since the Machine Learning (ML) approach was
adopted, significant progress in disease NER has
been achieved (Leaman 2009, Chowdhury 2010,
Neveol 2009). Robert et al. introduced the AZDC
corpus and adapted Conditional Random Fields
(CRF)-based gene mention recognition system,
BANNER for disease NER. They also combined the
CRF model with a dictionary-based method and
showed a significant improvement. Chowdhury et al.
studied different combination of feature sets, includ-
ing dictionary lookups and tags extracted by a syn-
tactic dependency parser. Those special feature
combinations in conjunction with carefully designed
postprocessing rules were observed to boost the
performance at a higher rate. However, incorpora-
tion of the domain dependent dictionary into a ML
system makes it non-trivial to adapt such a system
for another domain. This leads to an individual sys-
tem that is only applicable to a particular NER task
(such system might only address to gene mention
recognition problem) rather a unified system that
251
Munkhdalai T., Li M., Batsuren K. and Ryu K..
Towards a Unified Named Entity Recognition System - Disease Mention Identification.
DOI: 10.5220/0005287802510255
In Proceedings of the International Conference on Bioinformatics Models, Methods and Algorithms (BIOINFORMATICS-2015), pages 251-255
ISBN: 978-989-758-070-3
Copyright
c
2015 SCITEPRESS (Science and Technology Publications, Lda.)

could be applied to multiple NER tasks, such as
gene, chemical and disease mention recognition.
Recently, Semi-Supervised Learning (SSL) tech-
niques have been applied to NER. SSL a ML ap-
proach that typically uses a large amount of unla-
beled and a small amount of labeled data to build a
more accurate classification model than that which
would be built using only labeled data. SSL has
received significant attention for two reasons. First,
preparing a large amount of data for training re-
quires a lot of time and effort. Second, since SSL
exploits unlabeled data, the accuracy of classifiers is
generally improved. There have been two different
directions of SSL methods, semi-supervised model
induction approaches which are the traditional
methods and incorporate the domain knowledge
from unlabeled data into the classification model
during the training phase (Munkhdalai 2012,
Munkhdalai 2010), and supervised model induction
with unsupervised, possibly semi-supervised feature
learning. The approaches in the second research
direction induce a better feature representation by
learning over a large unlabeled corpus. Recently, the
studies that apply the word representation features
induced on the large text corpus have reported im-
provement over baseline systems in many natural
language processing (NLProc) tasks (Turian 2010,
Huang 2012, Socher 2011).
In this study, we take a step towards a unified
NER system in biomedical, chemical and medical
domain. We evaluate generally applicable word
representation features automatically learnt by a
large unlabeled corpus for disease NER. The word
representation features include brown cluster labels
and Word Vector Classes (WVC) built by applying
k-means clustering to continuous valued word vec-
tors of Neural Language Model (NLM). The exper-
imental evaluation using Arizona Disease Corpus
(AZDC) showed that these word representation fea-
tures boost system performance significantly as a
manually tuned domain dictionary does. BANNER-
CHEMDNER (Munkhdalai 2013), a chemical and
biomedical NER system has been extended with a
disease mention recognition model that achieves a
77.84% F-measure on AZDC when evaluating with
10-fold cross validation method.
The rest of this paper is organized as follows.
Section 2 introduces the proposed methodology and
the stages in the disease NER pipeline. Section 3
reports the performance evaluation of the system
based on combination of word representation fea-
tures, and a comparison against the existing systems.
Finally, we summarize the main conclusions
achieved and present our future work direction.
2 DISEASE NAMED ENTITY
RECOGNITION
This section introduces a detail of the proposed dis-
ease NER pipeline. First, we per-form preprocessing
on MEDLINE and PMC document collection and
then extract two different feature sets, a base feature
set and a word representation feature set, in the fea-
ture processing phase. The unlabeled set of the col-
lection is fed to unsupervised learning of the feature
processing phase to build word classes. Finally, we
apply the CRF sequence-labeling method to the
extracted feature vectors to train the NER model.
These steps will be described in subsequent sections.
2.1 Preprocessing
First, the text data is cleansed by removing non-
informative characters and replacing special charac-
ters with corresponding spellings. The text is then
tokenized with BANNER simple tokenizer. The
BANNER tokenizer breaks tokens into either a con-
tiguous block of letters and/or digits or a single
punctuation mark. Finally, the lemma and the part-
of-speech (POS) information were obtained for a
further usage in the feature extraction phase. In
BANNER-CHEMDNER, BioLemmatizer (Liu
2012) was used for lemma extraction, which resulted
in a significant improvement in overall system per-
formance for biomedical and chemical NER.
In addition to these preprocessing steps, special
care is taken to parse the PMC XML documents to
get the full text for the unlabeled data collection.
2.2 Feature Processing
We extract features from the preprocessed text to
represent each token as a feature vector, and then an
ML algorithm is employed to build a model for
NER.
The proposed method includes extraction of the
baseline and the word representation feature sets.
The word representation features can be extracted by
learning on a large amount of text and may be capa-
ble of introducing domain background to the NER
model.
The entire feature set for a token is expanded to
include features for the surroundings with a two-
length sliding window. The word, the word n-gram,
the character n-gram, and the traditional orthograph-
ic information are extracted as the baseline feature
set. The regular expressions that reveal orthographic
information are matched to the tokens to give ortho-
graphic information.
BIOINFORMATICS2015-InternationalConferenceonBioinformaticsModels,MethodsandAlgorithms
252

For word representation features, we train Brown
clustering models (Brown 1992) and Word Vector
(WV) models (Collobert 2008) on a large PubMed
and PMC document collection. Brown clustering is
a hierarchical word clustering method, grouping
words in an input corpus to maximize the mutual
information of bigrams. The VW model is induced
via a neural language model and consists of n-
dimensional continuous valued vectors, each of
which represents a word in the training corpus. Fur-
ther, the word vectors are clustered using a K-means
algorithm to drive a Word Vector Class (WVC)
model. Since Brown clustering is a bigram model,
this model may not be able to carry wide context
information of a word, whereas the WVC model is
an n-gram model (usually n=5) and learns broad
context information from the domain corpus. We
drive the cluster label prefixes with 4, 6, 10 and 20
lengths in the Brown model, 50 and 100 dimensions
of the WVs, and the WVCs as word representation
features.
2.3 Supervised Learning
CRF - a probabilistic undirected graphical model has
been used successfully in a large number of studies
on NER, because it takes advantage of sequence
labelling by treating each sentence as a sequence of
tokens. We apply a second-order CRF model, where
the current label is conditioned on the previous two
using a Begin, Inside, Outside (BIO) tagging format
of the tokens. In the BIO tagging format, each token
is classified either at the beginning, inside or outside
of a named entity, and a postprocessing task forms
the named entity mentions by merging the tagged
tokens.
We use a Machine Learning for Language
Toolkit (MALLET) library for training the CRF
model, because the BANNER system provides a
convenient interface to work with it. The BANNER
system also includes two types of general post-
processing that could be useful for any NER tasks in
bio-text data. The first type is based on the sym-
metry of parenthesis, brackets or double quotation
marks. Since these punctuation marks are always
paired, BANNER drops any named entity mention
containing mismatched parentheses, brackets or
double quotation marks. The second type of post-
processing is dedicated to resolving abbreviations of
named entities.
3 RESULTS
First, we evaluated combination of word representa-
tion features using 10-fold cross validation. We then
compared the result against existing systems
3.1 Dataset
We evaluated the system using AZDC corpus
(Leaman 2009) for disease mention identification.
The dataset consists of 793 annotated abstracts con-
taining 2,873 sentences, 3,093 unique disease men-
tions.
For the unlabeled data, we collected around 1.4
million PubMed abstracts and full text articles from
the whole PMC database available at the time (over
2 million documents). After preprocessing, we de-
rived two different text corpora: a PubMed abstract
corpus consisting of a vocabulary of 1,136,085 en-
tries for induction of Brown clustering models, and a
merged corpus of both resources with a vocabulary
of 4,359,932 entries for training WV models. Given
the limited resources and time, we were able to in-
duce the Brown clustering models only with the
PubMed abstract corpus.
3.2 Performance Evaluation
We followed an experimental setting similar to the
one in Robert et al. in order to compare our results
with that of the BANNER system. We performed
10-fold cross validation on AZDC in such a way that
all sentences of the same abstract are included in the
same fold. The results of the ten folds are averaged
to obtain the final outcome.
Table 1 shows the performance comparison of
the different runs with varied feature settings. We
started conducting a run with a basic feature setting,
and gradually increased the complexity of the fea-
ture space for further runs. A Brown model with a
larger number of clusters tended to obtain a higher
F-measure. Unlike Brown clustering, a large or a
lower number of WVCs degraded the performance.
We found the WVC model with 300 different clas-
ses the best performing one on this task. Further, the
combination of the different WVC models signifi-
cantly improved the F-measure. We achieved the
best performance, a 77.84% F-measure with the
model based on the baseline feature set, the 1000-
Brown clustering, and 300, 500 and 1000 WVCs
(the baseline + Brown 1000 + WVC 300 + WVC
500 + WVC 1000 setup).
TowardsaUnifiedNamedEntityRecognitionSystem-DiseaseMentionIdentification
253
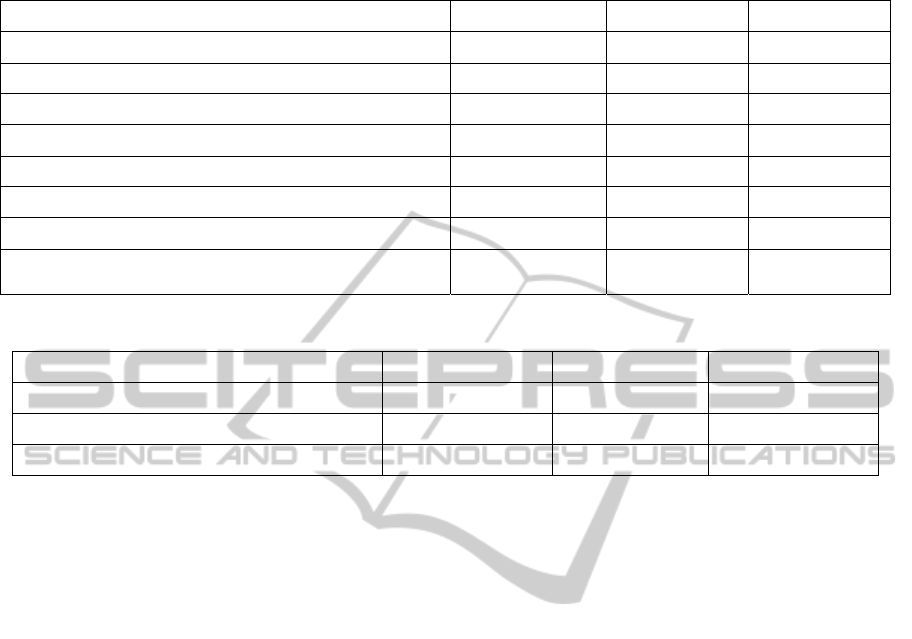
Table 1: Disease NER evaluation results of different runs with varied features. Feature groups are separated by (+) and
followed by the corresponding parameters.
Features Precision (%) Recall (%) F-score (%)
Baseline + Brown 300 78.96 72.41 75.53
Baseline + Brown 1000 78.93 73.55 76.1
Baseline + Brown 1000 + WVC 1000 79.6 73.59 76.45
Baseline + Brown 1000 + WVC 300 79.63 75 77.21
Baseline + Brown 1000 + WVC 500 78.88 74.39 76.54
Baseline + Brown 1000 + WVC 500 + WVC 300 80.06 74.66 77.25
Baseline + Brown 1000 + WVC 500 + WVC 1000 79.29 74.04 76.54
Baseline + Brown 1000 + WVC 500 + WVC 300 +
WVC 1000 80.44 75.45 77.84
Table 2: Comparison of BANNER and our system results.
Systems Precision (%) Recall (%) F-score (%)
BANNER 78.5 69.9 74
BANNER (with dictionary) 80.9 75.1 77.9
BANNER-CHEMDNER 80.44 75.45 77.84
3.3 Performance Comparison
Table 2 reports the comparison of BANNER and our
system results. Our system outperforms the basic
BANNER setup by a 3.84% F-measure. BANNER
combined with dictionary matching performs slight-
ly better than our system. Our system achieves a
higher recall, since it is based on ML. In contrast,
the precision of BANNER with dictionary is better.
In fact, this is the main advantage of dictionary-
based methods.
In our system, we do not rely on any lexicon nor
any dictionary other than the free text in the domain
in order to keep the system applicable to other NER
tasks in bio-text data, even though the usage of such
resources is reported to considerably boost system
performance.
4 CONCLUSIONS
We took a step towards a unified named entity
recognition system in biomedical, chemical and
medical domain. We evaluated word representation
features automatically learnt by a large unlabeled
corpus for disease named entity recognition system.
The word representation features include brown
cluster labels and word vector classes built by apply-
ing k-means clustering to continuous valued word
vectors of neural language model.
The experimental evaluation using Arizona dis-
ease corpus showed that these word representation
features boost system performance significantly as a
manually tuned domain dictionary does. BANNER-
CHEMDNER, a chemical and biomedical named
entity recognition system has been extended with a
disease mention recognition model that achieves a
77.84% F-measure on Arizona disease corpus when
evaluating with 10-fold cross validation method.
ACKNOWLEDGEMENTS
This research was supported by the Basic Science
Research Program through the National Research
Foundation of Korea (NRF) funded by the Ministry
of Science, ICT and Future Planning (No-
2013R1A2A2A01068923) and by a National Re-
search Foundation of Korea (NRF) grant funded by
the Korea government (MSIP) (No. 2008-0062611).
REFERENCES
Leaman, R., Gonzalez, G., 2008. Banner: An Executable
Survey of Advances in Biomedical Named Entity
Recognition. In Pacific Symposium on Biocomputing.
Munkhdalai, T., Li, M., Batsuren, K., Ryu, K. H., 2013.
Banner-Chemdner: Incorporating Domain Knowledge
BIOINFORMATICS2015-InternationalConferenceonBioinformaticsModels,MethodsandAlgorithms
254

in Chemical and Drug Named Entity Recognition. In
Fourth BioCreative.
Karopka, T., Fluck, J., Mevissen, H., Glass, A., 2006. The
autoimmune Disease Database: a dynamically com-
piled literature-derived database. BMC Bioinformatics.
Jimeno, A., Jimenez-Ruiz, E., Lee, V., Gaudan, S., Ber-
langa, R., Rebholz-Schuhmann, D., 2008. Assessment
of disease named entity recognition on a corpus of an-
notated sentences. BMC Bioinformatics.
Gurulingappa, H., Klinger, R., Hofmann-Apitius, M.,
Fluck, J., 2010. An Empirical Evaluation of Resources
for the Identification of Disease and Adverse Effects
in Biomedical Literature. In 2nd Workshop on Build-
ing and Evaluating Resources for Biomedical Text
Mining.
Leaman, R., Miller, C., 2009. Enabling Recognition of
Disease in Biomedical Text with Machine Learning:
Corpus and Benchmark. In Symposium on Languages
in Biology and Medicine.
Chowdhury, M. F. M., Lavelli, A., 2010. Disease Mention
Recognition with Specific Features. In Biomedical
Natural Language Processing.
Neveol, A., Kim, W., Wlbur, W. J., Lu, Z., 2009. Explor-
ing Two Biomedical Text Genres for Disease Recog-
nition. In Biomedical Natural Language Processing.
Munkhdalai, T., Li, M., Kim, T., Namsrai, O., Seon-phil,
J., Jungpil, S., Ryu, K. H., 2012. Bio Named Entity
Recognition based on Co-training Algorithm. In AINA
2012.
Munkhdalai, T., Li, M., Unil, Y., Namsrai, O., Ryu, K. H.,
2012. An Active Co-Training Algorithm for Biomedi-
cal Named-Entity Recognition. KIPS.
Turian, J., Ratinov, L., Bengio, Y., 2010. Word represen-
tations: A simple and general method for semi-
supervised learning. In ACL.
Huang, E. H., Socher, R., Manning, C. D., Ng, A. Y.,
2012. Improving Word Representations via Global
Context and Multiple Word Prototypes. In ACL.
Socher, R., Lin, C. C, Ng, A. Y., Manning, C. D., 2011.
Parsing Natural Scenes and Natural Language with
Recursive Neural Networks. In ICML.
Liu, H., Christiansen, T., Baumgartner, W. A., Verspoor,
K., 2012. BioLemmatizer: a lemmatization tool for
morphological processing of biomedical text. J. Bio.
Sem.
Brown, P. F., deSouza, P. V., Mercer, R. L., Pietra, V. J.
D., Lai, J. C., 1992. Class-Based n-gram Models of
Natural Language. In ACL.
Collobert, R., Weston, J., 2008. A Unified Architecture for
Natural Language Processing: Deep Neural Networks
with Multitask Learning. In ICML.
TowardsaUnifiedNamedEntityRecognitionSystem-DiseaseMentionIdentification
255
