
Sensor Array based on Metal Oxide Semiconductors for Detecting
Gas Mixtures and Its Sensing Properties
Byung-Min Kim and Jung-Sik Kim
Department of Materials Science and Engineering, The University of Seoul, Seoul, 130-743, Korea
Keywords: Gas Sensor Array, Gas Mixture, Metal Oxide Semiconducting Materials, MEMS Micro Sensor.
Abstract: Metal oxide semiconductor (MOS) gas sensors are very attractive owing to their low cost simplicity of use,
large number of detectable gases and various potential application fields. However, the MOS gas sensor has
a serious shortcoming of low selectivity in a mixture of gases, In this study MOS micro gas sensors were
fabricated for detecting carbon monoxide (CO), nitrogen oxide (NO
2
), ammonia (NH
3
) and formaldehyde
(HCHO) gases, as well as their binary mixed gas systems. Four sensing materials, Pd-SnO
2
for CO, In
2
O
3
for NO
X
, Ru-WO
3
for NH
3
, and SnO
2
-ZnO for HCHO were synthesized using a sol-gel method and
deposited in the middle of sensor platform. The micro gas sensor platform was fabricated by using a MEMS
technology. The sensing electrode and micro heater were designed to be a co-planar type structure with the
Pt thin film layer. The gas sensitivity and sensing behaviour for gas mixtures suggested that the selective
adsorption of one gas with respect to others occurred for gas mixture and resulted in good selectivity for a
particular gas species. Furthermore, the careful pattern recognition of sensing data obtained with sensor
array makes it possible to distinguish a gas species from gas mixture and to measure its concentration.
1 INTRODUCTION
Metal oxide semiconductor (MOS) gas sensors are
some of the most studied groups of gas sensors
owing to their low cost, simplicity of use, and large
number of detectable gases and various potential
application fields. On the other hand, MOS gas
sensors have serious shortcomings of their low
selectivity, response drifts and environmental
influences such as temperature, vibrations and the
gas flow (Korotcenkov, 2005). For practical
applications, MOS gas sensors have four major
issues of concern: selectivity, long-term stability,
reproducibility of the devices, and sensitivity.
Regarding the selectivity issue, the electronic nose
(e-nose) concept has been developed to achieve the
ability of classifying complex gas mixtures, such as
aromas and odors, using cross-sensitive sensors
(Weimar, 1998). In general, an e-nose system
utilizes gas sensing signals within the sensor array,
and the characteristics of individual sensors should
be as diverse as possible to ensure that the partial
sensor gas responses are not correlated for the
reliable discrimination of a certain gas from gas
mixture. As other issues related to the long-term
stability and reproducibility, such e-nose systems
require good reproducibility of the sensor array and
high training cost for sensor maintenance. This
appears to be one of key challenges requiring the
breakthrough (Eranna, 2004).
Gas identification techniques have attracted
considerable attention over the past twenty years.
The ability to monitor the leakage of combustible
and explosive gases is essential for preventing
accidental explosions and problems with the
pollution and the toxicity. Accordingly, there is
urgent demand for sensors combined with pattern
recognition systems that can detect and determine
the various kinds of combustible gases selectively
(Zhang, 2008).
In previous studies, we developed
four different MEMS-type gas sensors for the
detection of carbon monoxide (CO) (Kim, 2007),
nitrogen oxides (NO
x
) (Yoon, 2009), ammonia
(NH
3
) (Lee, 2010), and formaldehyde (HCHO) (Kim,
2012). Four sensing materials with nano-sized
particles for these target gases (Pd-SnO
2
nano-
powder for CO, In
2
O
3
nano-particle for NO
X
, Ru-
WO
3
nano-composite for NH
3
, and hybridized SnO
2
-
ZnO material for HCHO) were synthesized using a
sol-gel method. Each MEMS gas sensor showed
good sensing performance for its target gas, and the
optimal operating temperature was determined.
Kim, B-M. and Kim, J-S.
Sensor Array based on Metal Oxide Semiconductors for Detecting Gas Mixtures and Its Sensing Properties.
DOI: 10.5220/0005738201690174
In Proceedings of the 5th International Confererence on Sensor Networks (SENSORNETS 2016), pages 169-174
ISBN: 978-989-758-169-4
Copyright
c
2016 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
169

In this study, micro-hotplate-based MOS gas
sensors, which have a number of interesting features
and are particularly attractive for their practical
approach, were utilized for the detection of gas
mixtures. The sensing responses of four sensors
were investigated for the gas mixture, particularly
binary mixed gases along CO, NO
x
, NH
3
, and
HCHO. Then, selectivity and response pattern for
these sensors to a particular gas along mixed gases
were examined.
2 EXPERIMENTAL
2.1 Preparation of Sensing Materials
The appropriate sensing materials for four different
gases; SnO
2
for CO (designated as SN sensor), In
2
O
3
for NO
X
(IN sensor), WO
3
for NH
3
(WO sensor),
and SnO
2
-ZnO for HCHO (SZ sensor), were
synthesized using the sol-gel based method. Figure 1
shows field emission scanning electron microscopy
(FESEM) images of the four sensing materials. The
average particle sizes were 40 nm for SN, 70 nm for
IN, 1,000 nm for WO, and 20 nm for SZ sensors,
respectively.
(a) (b)
(c) (d)
Figure 1: Micro-structures of the four gas sensing
materials: (a) Pd-SnO
2
, (b) In
2
O
3
, (c) Ru-WO
3
, and (d) Pd
doped SnO
2
-ZnO.
Each sol-precusor containing its sensing element
was dripped with a micro-pipet on the electrode of
sensor platform, and then heat-treated at its
appropriate sintering temperature. Table 1 represents
main features of sensing materials and the optimum
temperature for their gas sensing.
Table 1: Main features of four different sensing materials.
Composition Average
Particle Size
Optimum
Temp. (°C)
SN 1% Pd-SnO
2
40 nm 225
IN In
2
O
3
70 nm 225
WO 1% Ru-WO
3
1.0 μm 367
SZ 1% Pd + SnO
2
-ZnO 20 nm 367
2.2 Fabrication of MEMS-based
Sensors
Micro gas sensor platforms were designed with co-
planar type in which sensor electrode and micro-
heater were existed on the identical film (Pt thin
film) layer, and fabricated using the MEMS process,
previously (Choi, 2012). The sensor chip size of the
MEMS platform was 1.8 mm × 1.8 mm, and the
membrane located in the central part of the sensor
chip was 0.9 mm × 0.9 mm. Figure 2(a) shows the
photograph of the IN sensor device with TO-39
package in which the sensor chip was placed and
connected to the electric terminals by Au wires. The
fabricated sensor had low power dissipation, and its
power consumption increased linearly with
increasing operation temperature as shown in Figure
2 (b). For example, power consumptions operated at
225°C for the SN sensor and 367°C for the WO
sensor were 35.26 and 64.37 mW, respectively.
(a)
(b)
Figure 2: (a) Photograph of fabricated sensor on the TO-
39 package and (b) electro-thermal characteristic as the
heating power vs. operating temperature.
SENSORNETS 2016 - 5th International Conference on Sensor Networks
170

2.3 Measurement of Sensing Response
The sensing properties were tested for gas mixture in
a gas chamber in which four gas sensors were placed.
The gas chamber was connected to a computer-
supervised continuous gas flow system that
produced the desired concentration for each gas and
gas mixtures with a good reproducibility. The test
gases (CO, NO
X
, NH
3
, and HCHO) were diluted
with a nitrogen gas and carried by dry air at a
constant flow rate. The total gas flow rate was about
500 ml/min. The concentration of each test gas was
0 ~ 60 ppm for CO, 0~0.6 ppm for NO
2
, 0 ~ 10.0 ppm
for NH
3
, and 0 ~ 5.0 ppm for HCHO, respectively.
To quantify the sensor response for both oxidizing
and reducing gases as well as their mixtures, the gas
sensitivity (S) was defined as S = log (R
g
/R
a
), where
R
a
is the sensor resistance in air and R
g
is the sensor
resistance after injecting the test gas. The gas
sensitivity showed negative values (S < 0) for
reducing gases, and positive values (S > 0) for
oxidizing gases because all sensors were
simultaneously sensitive to both reducing (CO, NH
3
and HCHO) and oxidizing (NO
2
) gases. The gas
sensing properties and selective reactions to several
gases were analyzed by quantifying the sensitivity.
3 RESULTS AND DISCUSSION
3.1 Sensing Response for CO-NO
2
Gas
Mixture
Figure 3 shows the variations of the gas sensitivity
of all sensors to CO and NO
2
gases and their mixture.
The SN and IN sensors showed stronger responses
to NO
2
gas than CO, and their sensitivities showed
positive values in the presence of NO
2
gas. The SN
sensor exhibited a strong response to both gases and
their mixtures, whereas the IN sensor responded
only to NO
2
. The WO and SZ sensors exhibited
similar behaviors to the SN sensor, but their
sensitivities to NO
2
gas were slightly lower.
(a)
(b)
(c)
(d)
Figure 3: Gas sensing properties in the CO-NO
2
system;
(a) SN, (b) IN, (c) WO, and (d) SZ sensors.
3.2 Sensing Response for CO-NH
3
Gas
Mixture
In the CO-NH
3
gas mixture, the CO gas responses
were much higher than NH
3
gas in a mixture of
reducing agents, but the sensitivities in the gas
mixture were higher than that to each gas separately
under most experimental conditions (Figure 4). The
SN and SZ sensors were more sensitive to their
target gas (CO) than NH
3
within the test ranges: S =
-0.179 and -0.420 for SN, and S = -0.100 and -0.176
for SZ sensors (at CO 30 ppm and 60 ppm). On the
other hand, the changes in resistance were slightly
lower in the case of a gas mixture. The IN sensor
responses were quite poor to both CO and NH
3
gases.
The WO and SZ sensors were sensitive to both the
single gases and their mixtures, with higher
sensitivities observed with the gas mixtures. For the
tests in a mixture of reducing agents, the sensor
responses targeting these gases did not show any
synergic effects. The sensitivity of the SN sensor
was -0.420 for 60 ppm CO gas, but the sensitivity
Sensor Array based on Metal Oxide Semiconductors for Detecting Gas Mixtures and Its Sensing Properties
171
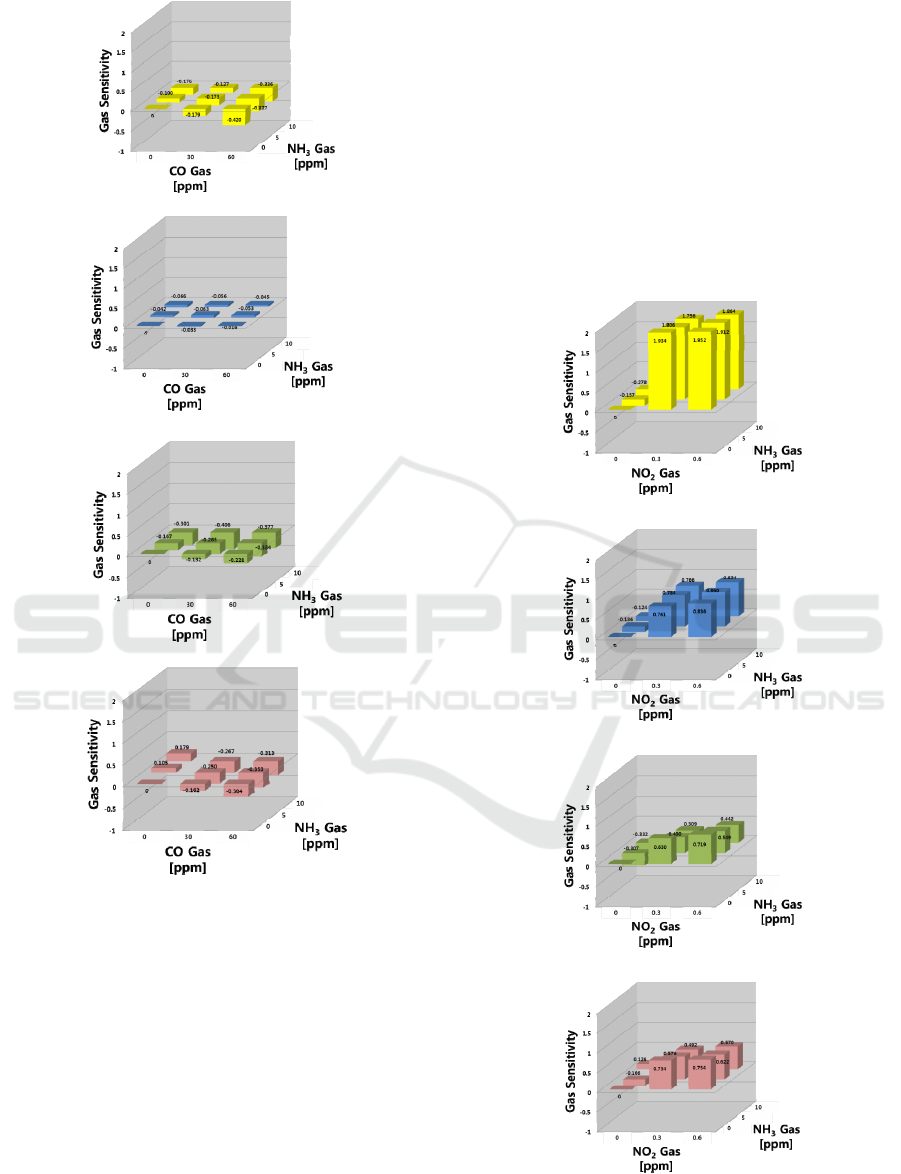
(a)
(b)
(c)
(d)
Figure 4: Gas sensing properties in the CO-NH
3
system;
(a) SN, (b) IN, (c) WO, and (d) SZ sensors.
was slightly lower (S = -0.327) for CO 60 ppm –
NH
3
2.5 ppm. This phenomenon was not observed in
the other sensors, which had gas selectivity for the
specific gas species. In three mixtures (CO 60 ppm –
NH
3
0 ppm, CO 60 ppm – NH
3
5.0 ppm, and CO 60
ppm – NH
3
10.0 ppm), the CO concentration was
identical. As the NH
3
concentration increased,
however, the sensitivity of the SN sensor was
slightly lower for the gas mixtures than for the single
CO gas. This suggests that the specific adsorption
and selective activation of adsorption sites might
occur in gas mixtures and offer a priority for the
adsorption of a specific gas, which will be discussed
in the following section.
3.3 Sensing Response for NO
2
-NH
3
Gas Mixture
In the NO
2
-NH
3
gas mixture, as shown in Figure 5,
the responses to NO
2
gas were stronger than those of
NH
3
. In the SN sensor, the sensitivities exhibited
increasing behavior to NO
2
and decreasing behavior
to NH
3
at higher concentrations, showing that the
sensor responds to both gases (S = 0.934 at 0.3 ppm).
(a)
(b)
(c)
(d)
Figure 5: Gas sensing properties in the NO
2
-NH
3
system;
(a) SN, (b) IN, (c) WO, and (d) SZ sensors.
SENSORNETS 2016 - 5th International Conference on Sensor Networks
172
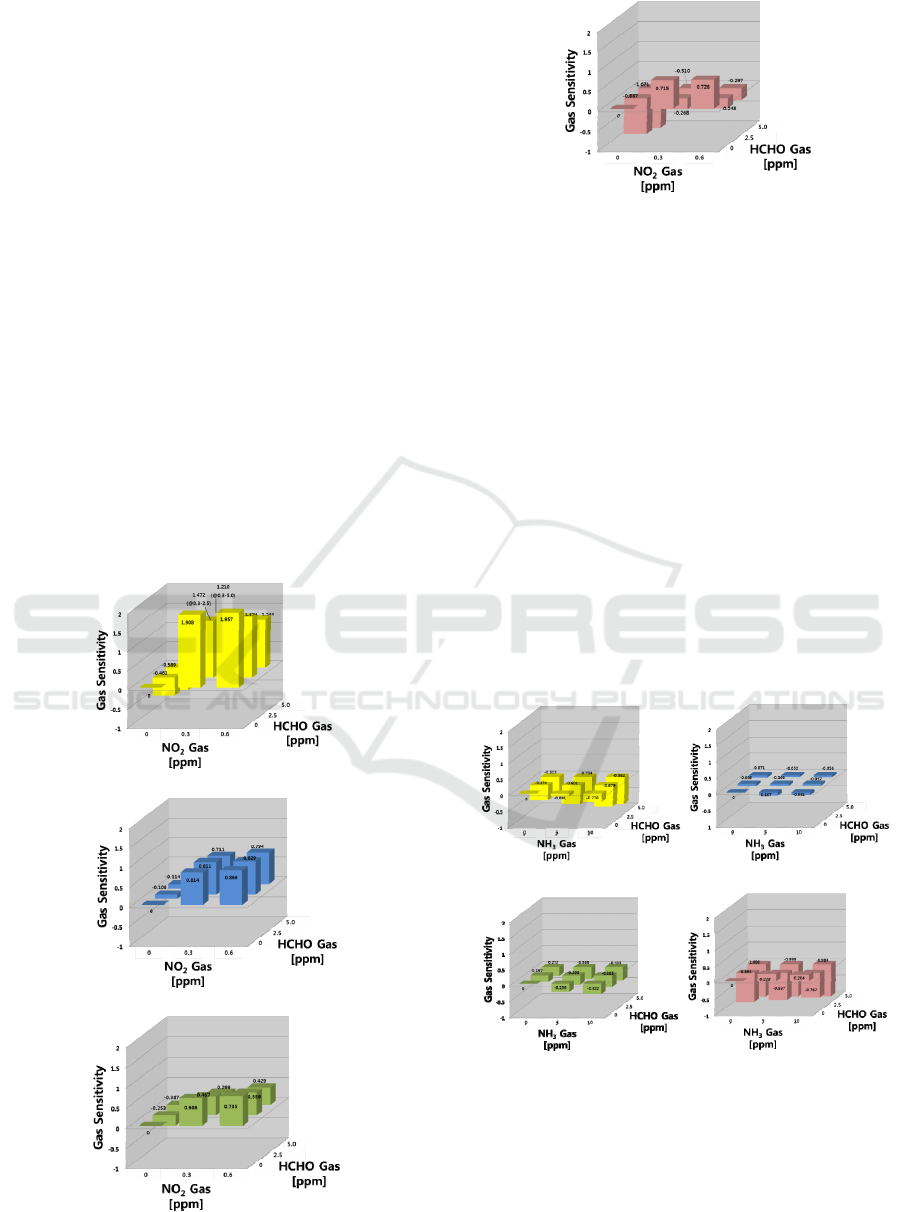
In the gas mixtures, the changes in resistance were
higher than the baseline (Ra) and decreased with
increasing NH
3
concentration. On the other hand, the
sensitivities to both gases had positive values (S > 0)
within the test ranges. The IN sensor had a selective
response to NO
2
gas but was barely sensitive to NH
3
gas. As the NH
3
gas concentration increased, the IN
sensor exhibited a slight decrease in sensitivity to
NO
2
gas in the NO
2
-NH
3
mixed gas system. The
WO sensor showed similar behaviour to the IN
sensor, but it was a little more sensitive to NO
2
gas.
3.4 Sensing Response for NO
2
-HCHO
Gas Mixture
In the NO
2
-HCHO system, the responses to NO
2
gas
were stronger than those of HCHO (in Figure 6). In
the SN and WO sensors, the sensitivities showed
increasing behavior to NO
2
and decreasing behavior
to HCHO at higher concentrations. On the other
hand, the IN sensor is selective to NO
2
gas, since it
shows no response to HCHO gas. As the HCHO gas
concentration increased, the IN sensor showed a
slight decrease in sensitivity to NO
2
gas in the NO
2
-
(a)
(b)
(c)
(d)
Figure 6: Gas sensing properties in the NO
2
-HCHO
system; (a) SN, (b) IN, (c) WO, and (d) SZ sensors.
HCHO mixed gas system. The WO sensor showed
similar behavior to the IN sensor, as it is selective to
NO
2
gas. The SZ sensor responses toward HCHO
were stronger than NO
2
gas for the NO
2
and HCHO
gas mixture.
3.5 Sensing Response for NH
3
-HCHO
Gas Mixture
For the NH
3
-HCHO system as shown in Figure 7,
the IN sensor did not respond to any of gases; NH
3
and HCHO, three other sensors (SN, WO and SZ)
were sensitive to either single gas or its mixture. The
SZ sensor is selective to HCHO. In this mixture of
two reducing gases, there was no synergic effect in
the SN sensor responding to both NH
3
and HCHO
gases.
(a) (b)
(c) (d)
Figure 7: Gas sensing properties in the NH
3
-HCHO
system; (a) SN, (b) IN, (c) WO, and (d) SZ sensors.
3.6 Discussion
The present study analyzed the sensing properties of
micro gas sensor arrays by examining the raw data
Sensor Array based on Metal Oxide Semiconductors for Detecting Gas Mixtures and Its Sensing Properties
173

in the gas mixtures. Overall, the IN sensor could
detect NO
2
selectively, whereas the SN sensors
detected all four gases (CO, NO
2
, NH
3
and HCHO).
If coupled with an IN sensor, SN is capable of
detecting NO
2
sensitively. On the other hand, the gas
sensitivity signals of the two sensors were not
sufficient for the detection of all four gases. The WO
and SZ sensors detected all four gases but had low
gas selectivity. Therefore, the four-sensor-array
would be sufficient to discriminate mixtures of these
gases. To gain clear insight into the applicability of
the sensor array in this application, the responses
with sensitivity can be arranged in a 4 × 4 matrix, in
which each element represents the response of each
sensor to each target gas. This matrix suggests how
the different gas contributions can be extrapolated
from the sensor array data using the signal process.
4 CONCLUSIONS
The sensing properties for the MEMS-based MOS
gas sensors were investigated with gas mixtures
along CO, NO
X
, NH
3
, and HCHO gases. Four
different gas sensors were fabricated for the
detection of CO, NO
X
, NH
3
, and HCHO gases,
respectively. Each sensor exhibited good sensitivity
to its target gas, and the optimum operating
temperature of micro-heater was examined. The
sensing response behaviors for gas mixture were
analyzed using the experimental data in the MEMS
gas sensor arrays with respect to selectivity and
response pattern. The gas sensing behaviors in
mixed gas systems suggest that specific adsorption
and selective activation of adsorption sites might
occur in gas mixtures and offer priority for the
adsorption of a specific gas. An analysis of the
sensing performance of the sensor arrays will make
it possible to discriminate the components in
harmful gas mixtures as well as their concentrations
using pattern recognition techniques.
ACKNOWLEDGEMENTS
This research was supported by the National
Research Foundation of Korea (NRF) funded by the
Korea government (MSIP) (No. 2015-
R1A2A2A01005790).
REFERENCES
Korotcenkov, G., 2005. Gas Response Control Through
Structural and Chemical Modification of Metal Oxide
Films: State of the Art and Approaches. Sens.
Actuators B, Vol. 107, pp.209-232.
Weimar, U., Gopel, W., 1998. Chemical Imaging. Part II:
Trends in Practical Multiparameter Sensors Systems.
Sens. Actuators B, Vol. 52, pp.143-161.
Eranna, G, Joshi, B. C., Runthala, D. P., Gupta, R. P.,
2004. Oxide Materials for Development of Integrated
Gas Sensors-A Comprehensive Review. Critical
Reviews in Solid State Materials Sciences, Vol. 29,
pp.111-188.
Zhang, S., Xie, C., Zeng, D., Li, H., Bai, Z., Cai, S., 2008.
A Method of Feature Extraction from the Desorption
Part of MOX’s Response Curves to Gases. IEEE Sens.
J., Vol. 8, pp.1816-1823.
Kim, S. D., Kim, B. J., Yoon. J. H., Kim, J. S., 2007.
Design, Fabrication and Characterization of a Low-
Power Gas Sensor with High Sensitivity to CO Gas. J.
Kor. Phys. Soc., Vol. 51, pp.2069-2076.
Yoon, J. H., Kim, J. S., 2009. Design and Fabrication of a
MEMS-Based Gas Sensor. Adv. Mater. Res., Vol. 74,
pp.255-258.
Lee, H. J., Yoon, J. H., Kim, B. J., Jang, H. D., Kim, J. S.
2010. Gas Sensing Characteristics of Ru Doped-WO
3
Micro Gas Sensors. Kor. J. Met. Mater., Vol. 49,
pp.395-399.
Kim, B. J., Lee, H. J., Yoon, J. H., Kim, J. S., 2012. Highly
Sensitive Formaldehyde Gas Sensors Based on SnO
2
-
ZnO Nanocomposites. Sens. Lett., Vol. 10, pp.1-7.
Choi, W. S., Kim, B. J., Lee, H. J., Choi, J. W., Kim, S. D.,
Min, N. K., 2012. Study on the Micro-Heater
Geometry in MEMS Gas Sensor Platforms and Effects
on Gas Detecting Performances. J. Nanosci. Nanotech.,
Vol. 12, pp.1-4.
SENSORNETS 2016 - 5th International Conference on Sensor Networks
174
