
Investigation of N-Butanol Blending with Gasoline using a 1-D
Engine Model
Simeon Iliev
University of Ruse “Angel Kanchev”, Department of Engines and Vehicles, 8 Studentska Str., Ruse, Bulgaria
Keywords: Alternative Fuels, Butanol Blends, Engine Simulation, Gasoline, Internal Combustion Engine.
Abstract: Increasing demand and limited reserves for fossil fuel together with carbon emissions regulations have led to
producing sustainable fuels made from renewable materials. In recent years, the focus has been on using bio-
fuels as alternate energy sources. Blending bio-fuels with gasoline is one of the methods to be considered
under the search for a new source of energy. Alcohols are an important category of bio-fuels. Butanol can be
an alternative fuel since it is a liquid and has several physical and chemical properties similar to those of
gasoline fuels. Butanol don’t have many of the drawback associated with ethanol. Butanol has also a higher
molecular weight than ethanol, and therefore, has reduced vapour pressure, lower water solubility, and higher
energy density. That is why this study is aimed to develop the 1-D model of a PFI (Port Fuel Injection) engine
for predicting the effect of various blends of butanol and gasoline on engine performances and fuel
consumption. AVL Boost was used as a simulation tool to analyze the performance and emissions for different
blends of n-butanol and gasoline by volume (n-B0, n-B5, n-B10, n-B20, n-B30, n-B50 and n-B85).
1 INTRODUCTION
Ethanol, butanol and biodiesel are main biofuels.
Butanol (butyl alcohol) is a liquid alcohol fuel and
can work in the internal combustion engine with
gasoline without any modification. It can be produced
from biomass (biobutanol) or from fossil fuels
(petrobutanol). Both alcohols biobutanol and
petrobutanol have the same chemical properties. The
energy density of butanol is closer to gasoline than
the other alternative additives as ethanol and
methanol which are commonly used today. Butanol is
less hygroscopic so it does not require the different
handling that ethanol and methanol required. Also, it
means that Butanol is less corrosive than ethanol and
methanol. In comparison to ethanol, butanol is less
prone to water contamination. As a result it could be
distributed using the same infrastructure used to
transport gasoline. Butanol can burn at a wider range
of temperatures than ethanol, and has better cold start
properties. Many investigations lead to conclusion
that that it can be used alone or can be mixed with
gasoline in an internal combustion engine (ICE).
Furthermore, butanol has a high enough octane
number, close to that of gasoline and a lower vapor
pressure. The higher octane number, the more
compression the fuel can withstand before detonating.
Premature fuel ignition can damage engine, which is
a common phenomenon for lower octane number
fuel. These properties make it more suitable additive
than ethanol and methanol for gasoline fuel.
There are four butyl alcohols with the same
chemical composition consisting of 4 carbon atoms,
10 hydrogens and 1 oxygen and they have identical
chemical pattern C
4
H
10
O, but they differ each from
others with respect to their structure (Szwajaa and
Naber, 2010). The chemical nature of alcohols are as
follows:
1-butanol (n-butanol, n-butylalcohol)
CH
3
(CH
2
)
2
CH
2
OH,
2-butanol CH
3
CH(OH)CH
2
CH
3
,
3-butanol (CH
3
)
3
COH,
iso-butanol CH
3
(CH
2
)
3
OH.
The common fuel properties of n-butanol in
comparison to gasoline and other alcohol fuels are
given in Table 1 (Yacoub, Bara and Gautam 2000),
(Gautam and Martin, 2000). Form this table, it can be
said that about the latent heat of vaporization of these
fuels, butanol is less attractive than others. For PFI
(port fuel injection) systems, fuels with higher latent
heat of vaporization have larger decreases in
temperature of intake charge with complete
Iliev, S.
Investigation of N-Butanol Blending with Gasoline using a 1-D Engine Model.
DOI: 10.5220/0006284703850391
In Proceedings of the 3rd International Conference on Vehicle Technology and Intelligent Transport Systems (VEHITS 2017), pages 385-391
ISBN: 978-989-758-242-4
Copyright © 2017 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
385
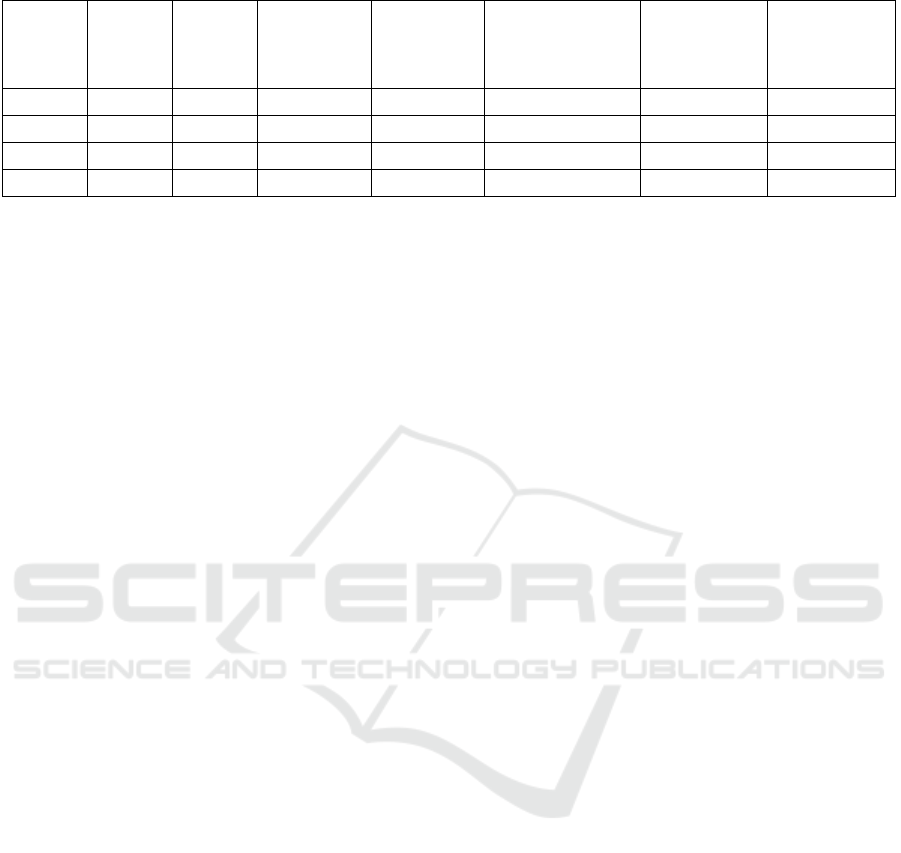
Table 1: Properties of several fuels (Yacoub, Bara and Gautam 2000), (Gautam and Martin, 2000).
Fuel Chemical
formula
Specific
gravity
(kg/dm
3
)
Lower heating
value
(MJ/kg)
Stoichiometri
c air–fuel
ratio
(kg
air
/kg
fuel
)
Energy density of a
stoichiometric air–
fuel mixture
(MJ/kg)
Latent heat of
vaporization (at
boiling point)
(kJ/kg)
Octane number
(RON+MON)/2
Methanol CH
3
OH 0,7913 20,08 6,43 2,750 1098 99
Ethanol C
2
H
5
OH 0,7894 26,83 8,94 2,699 838 100
n-Butanol C
4
H
9
OH 0,8097 32,01 11,12 2,641 584 86
Gasoline C
8
H
15
0,7430 42,9 14,51 2,769 349 87
vaporization in the intake port. To match the
combustion characteristics of gasoline, the utilization
of butanol fuel as a substitute for gasoline requires
fuel-flow increases (though butanol has only slightly
less energy than gasoline, so the fuel-flow increase
required is only minimal, maybe 10%). Higher
oxygen content and lower octane number of n-butanol
need changes in initial engine calibration, determined
with pure gasoline. Also butanol has a higher laminar
flame propagation speed than gasoline, which makes
combustion process finish earlier and improving the
engine thermal efficiency.
Since using blended alcohol-gasoline fuels can
reduce the air pollution, many researchers have
studied the effect of these alcohol blended fuels on
the performance and exhaust emission of a spark-
ignited engine. In а study conducted by (Alasfour,
1998), NOx emissions were presented as a function
of air/fuel equivalence ratio. It is showed decreasing
in NOx emissions when a 30% butanol-gasoline
blend was used. Peak NOx emissions were received
at a slightly leaner mixture for a 30% butanol–
gasoline blend than for pure gasoline.
Switching a gasoline engine over to butanol
would in theory result in a fuel consumption penalty
of about 10% but there is no scientific study yet which
butanol effects on fuel consumption for the
commercial vehicles. While the energy density for
any mixture of gasoline and butanol can be
calculated, tests with other alcohol fuels have
demonstrated that the effect on fuel economy is not
proportional to the change in energy density (Minter,
2006).
There are many investigations on butanol
utilization in gasoline engines. The researchers
(Wallner, Miers and McConnell, 2009; Rakopoulos,
Papagiannakis and Kyritsis, 2011; Sarathy et al.,
2009) investigated the unburned hydrocarbon (HC),
carbon monoxide (CO) and nitrogen oxides (NOx)
emissions with gasoline, 10% ethanol and 10% n-
butanol blends in a direct injection spark-ignition
engine. Their results showed little difference in HC,
CO and NOx emissions between gasoline and 10% n-
butanol. The reason for this is the engine is operated
at the stoichiometric air/fuel ratio for each specific
fuel blend. The researchers (Dernotte, et al., 2010)
examined the emissions characteristics of several n-
butanol–gasoline blends (0, 20, 40, 60 and 80 vol. %
of n-butanol in gasoline) using a PFI spark-ignition
engine and found that n-butanol (B60) and n-butanol
(B80) produced 18% and 47% more unburned HC
emissions than neat gasoline, respectively. It was
found that B80 was the only n-butanol blended fuel,
which it did not produce lower CO emissions than
neat gasoline.
Other researchers (Gu X et al., 2012) tested five
gasoline butanol blendes (B0, B10, B30, B40 and
B100) and results showed that the unburned HC and
CO emissions of blends are lower than those of
gasoline. Pure n-butanol (B100) increases the
unburned HC and CO emissions compared to those of
gasoline. They also showed that the addition of n-
butanol decreased the particle emissions. In another
study (Feng et al., 2013) for pure gasoline and 35%
by volume butanol-gasoline blend. The results
showed that engine torque, brake specific fuel
consumption (BSFC) and CO and HC emissions were
better than those of pure gasoline at both full load and
partial load with 35% volume butanol addition. But
CO
2
emission was worse than that of the original level
of pure gasoline.
There are many studies focused on conventional
harmful exhaust emissions (CO, HC and NOx) when
use butanol as spark ignition engine fuel. Although
CO
2
is a non-toxic gas, which is not classified as an
engine pollutant, it is one of the substances
responsible for global temperature rises through the
greenhouse effect. CO
2
emission has not been usually
taken into account in many studies. (Emilio et al.,
2013; Ritche et al., 2012).
The objective of this paper is to investigate CO,
HC, NOx, BSFC, power and torque for various n-
butanol-gasoline blends at diferent engine speed.
SMS 2017 - Special Session on Sustainable mobility solutions: vehicle and traffic simulation, on-road trials and EV charging
386
2 METODOLOGY
Engine simulation is becoming an increasingly
important engineering tool for time and cost
efficiency in the development of internal combustion
engines (ICEs). Most of results that are obtained by
simulation are rather difficult to be obtained
experimentally. The use of Computational Fluid
Dynamics (CFD) simulations allow researchers to
understand flow behaviour and quantify important
flow parameters such as mass flow rates or pressure
drops, provided that the CFD tools have been
properly validated against experimental results. For
reasons such as the aforementioned, CFD simulations
have become a valuable tool in helping both the
analysis and design of the intake and exhaust systems
of an ICEs. Many processes in the engine are 3-D but
it requires greater knowledge and large computational
time. Thus simplified 1-D simulation is often used.
There are several components that manifest a
complex three-dimensional flow behaviour, such as
turbo machinery or manifolds which cannot be
simulated properly by 1-D codes, and thus require
viscous, 3-D codes (Iliev S. 2015).
The present paper aims to develop the 1-D
simulation model of four-stroke port fuel injection
(PFI) gasoline engine for predicting the effect of n-
butanol–gasoline (n-B0, n-B5, n-B10, n-B20, n-B30,
n-B50 and n-B85) fuel blends on the performance and
emissions of SI engine. For this purpose, the
simulation of a calibrated gasoline engine model was
used as basic operating condition, and the laminar
burning velocity correlations of n-butanol–gasoline
fuel blends was considered for calculating the
different combustion duration. The engine
performances: torque and specific fuel consumption
were compared and discussed.
2.1 Simulation Setup
The 1-D engine simulation model is developed by
using the software AVL BOOST and has been
employed to study the engine performance working
on n-butanol-gasoline blends.
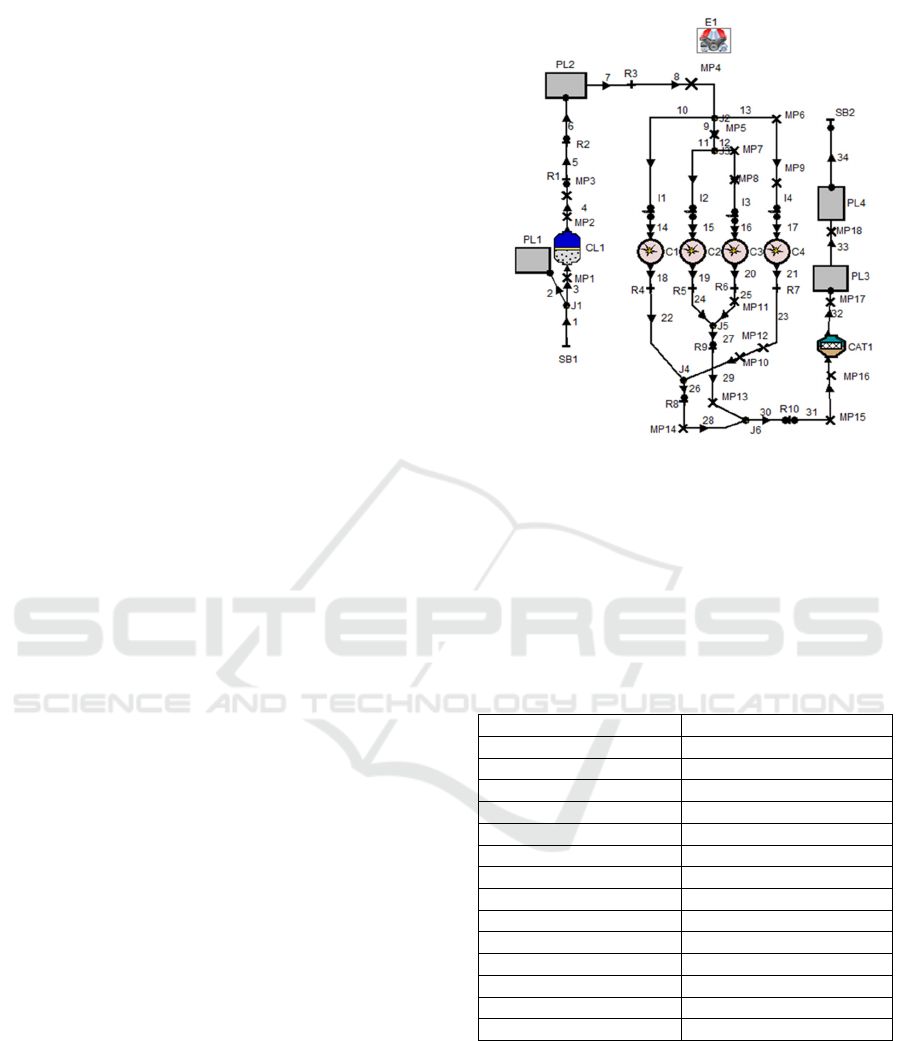
Figure 1: Layout of engine model.
The pre-processing step of AVL Boost enable
the user to model a 1-Dimensional (1-D) engine test
bench setup using the predefined elements provided
in the software toolbox. The various elements are
joined by the desired connectors to establish the
complete engine model using pipelines.
Table 2: Engine specification.
Engine parameters Value
Bore 86 (mm)
Stroke 86 (mm)
Compression ratio 10,5
Connection rod length 143,5 (mm)
Number of cylinder 4
Piston pin offset 0 (mm)
Displacement 2000 (cc)
Intake valve open 20 BTDC (deg)
Intake valve close 70 ABDC (deg)
Exhaust valve open 50 BBDC (deg)
Exhaust valve close 30 ATDC (deg)
Piston surface area 5809 (mm
2
)
Cylinder surface area 7550 (mm
2
)
Number of stroke 4
In Fig.1, E1 represent the engine while C1, C2,
C3 and C4 represent the number of cylinders of the
engine. MP1 to MP18 represent the measuring points.
PL1, PL2, PL3 and PL4 represent the plenum. SB1
and SB2 are for the system boundary. The flow pipes
are numbered 1 to 34. CL1 represent the cleaner. R1
to R10 represent flow restrictions, CAT1 represent
catalyst and I1 to I4 represent fuel injectors.
Investigation of N-Butanol Blending with Gasoline using a 1-D Engine Model
387
The engine model used in this simulation was
performed on a four stroke, four cylinder spark
ignition engine with port fuel injection. The gasoline
engine model was calibrated and described by (Iliev
S. 2014) and its layout is shown in Fig. 1 with engine
specification shown in Table 1.
3 RESULT AND DISCUSSON
The present study concentrated on the emission and
performance characteristics of the n-butanol-gasoline
blends. Different concentrations of the blends 0% n-
Butanol (n-B0), 5% n-Butanol (n-B5), 10% n-
Butanol (n-B10), 20% n-Butanol (n-B20), 30% n-
Butanol (n-B30), 50% n-Butanol (n-B50) and 85% n-
Butanol (n-B85) by volume were analyzed using
AVL BOOST at full load conditions for the speeds
ranging from 1000 - 6500 rpm in the steps of 500rpm.
The results are divided into different subsections
based on the parameter analyzed.
3.1 Engine Performance
Characteristics
The results of the brake power, and specific fuel
consumption for n-Butanol gasoline blended fuels at
different engine speeds are presented here.
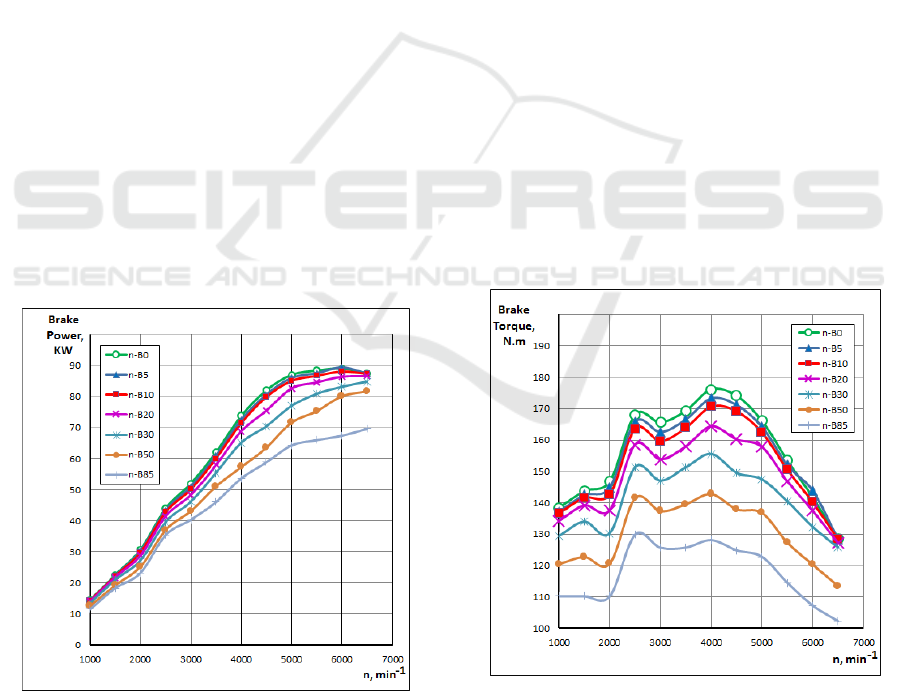
Fig. 2 shows the influence of n-Butanol gasoline
blended fuels on engine brake power.
Figure 2: Influence of n-Butanol gasoline blended fuels on
engine brake power.
The brake power is one of the important factors that
determine the performance of an engine. The
variation of brake power with speed was obtained at
full load conditions for n-B5, n-B10, n-B20, n-B30,
n-B50 and pure gasoline n-B0, using the CFD results.
When the n-Butanol content in the blended fuel
was increased, the engine brake power decreased for
all engine speeds. The brake power of gasoline was
higher than those of n-B5 to n-B85 for all engine
speeds. The heating value of n-Butanol is lower than
that of gasoline and heating value of the blended fuel
decreases with the increase of the n-Butanol content.
As a result, a lower power output is obtained.
Butanol addition to the gasoline does not affect
engine power significantly, but especially at high
engine speed (over 4000 min
-1
) there is a sharp
reduction the power curves compared to pure gasoline
(Fig. 2). The reason of this reduction can be affected
by the low calorific value of butanol.
This may refer to some reasons as follows.
Combustion characteristic of n-butanol is different
from gasoline since the latent heat of n-butanol is
higher than that for gasoline (584 kJ/kg, 349 kJ/kg for
n-butanol and gasoline, respectively). This means that
the n-butanol absorbs more heat in order to evaporate
and burn.
Fig. 3 shows the influence of n-Butanol gasoline
blended fuels on engine torque. The increase of n-
Butanol content (n-B5 – n-B85) decreased the torque
of the engine. The brake torque of gasoline was
higher than those of n-B5-nB85.
Figure 3: Influence of n-Butanol gasoline blended fuels on
engine torque.
The calorific value of butanol is lower than the
calorific value of gasoline, therefore it is expected
that any butanol addition to the gasoline reduces the
SMS 2017 - Special Session on Sustainable mobility solutions: vehicle and traffic simulation, on-road trials and EV charging
388
torque output of the engine. However oxygen content
of butanol improves the combustion in the cylinder
and with the 5% and %10 butanol blends similar
value are achieved with gasoline. On the other hand,
because of the existence of oxygen in the butanol
chemical component, and the increase of n-Butanol,
lean mixtures are produced that decrease equivalence
air-fuel ratio to a lower value and due to the presence
of oxygen which has entered the combustion chamber
makes the burning more efficient.
Regarding the stumpy of volumetric efficiency of
blended fuels, it is mainly due to the low saturation
pressure of n-butanol compared to gasoline fuel (2.27
kPa of n-butanol and 31 kPa for gasoline); the
saturation pressure is strongly linked to the ability of
the fuel to vaporize. The lower the saturation pressure
is, the ability of the fuel to evaporation is increased.
When fuel is evaporated, the volume of vaporized
fuel will displace some incoming air, e.g., less air.
Besides, fuels with a smaller stoichiometric air–fuel
ratio, like n-butanol 11.2, have a lower volumetric
efficiency. But, n-butanol has high heat of
vaporization, so some volumetric efficiency lost due
to air-fuel ratio and saturation pressure is partially
gained back again. Besides, it is possible to improve
the volumetric efficiency of n-butanol by cooling the
air and fuel before accessing onto the engine system.
In addition, manifolds with late fuel addition and
wider runners can be designed to further increase the
volumetric efficiency.
Figure 4: Influence of n-Butanol gasoline blended fuels on
brake specific fuel consumption.
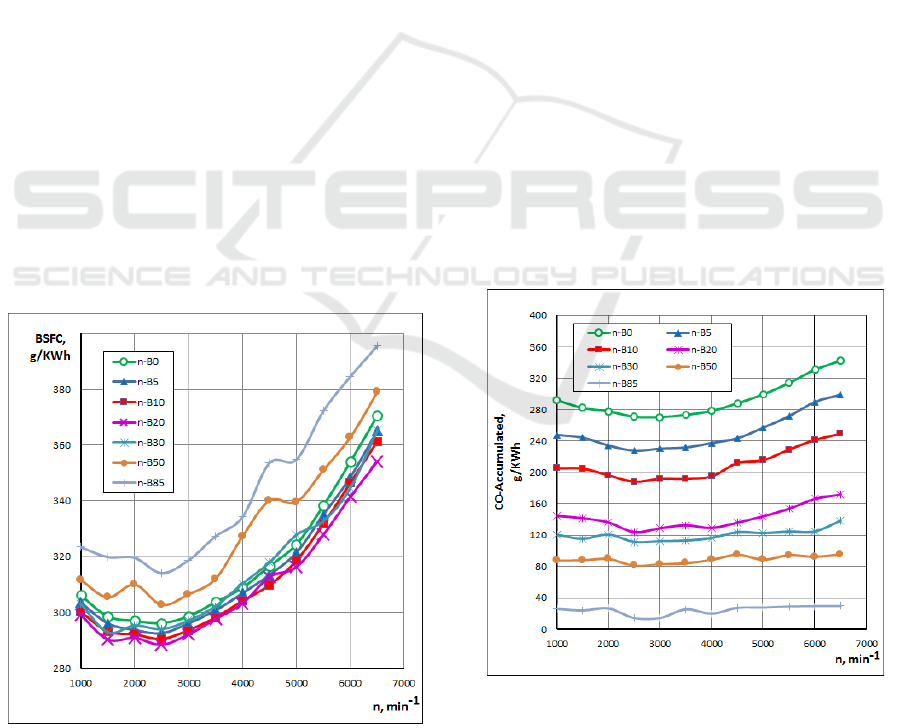
Fig. 4 indicates the variations of the BSFC for
n-Butanol gasoline blended fuels under various
engine speeds. As shown in this figure, the BSFC
increased as the n-Butanol percentage increased. The
well-known reason, that the lower heating value and
stoichiometric air-fuel ratio for this fuel leads that for
specific air-fuel equivalence ratio, more fuel is
needed. The highest specific fuel consumption is
obtained for n-B85 blended fuel. Also, a slight
difference exists between the BSFC when using
gasoline and when using n-Butanol gasoline blended
fuels (n-B5, n-B10 and n-B20). The lower energy
content of butanol gasoline blended fuels causes some
increment in BSFC of the engine when it is used
without any modification.
3.2 Engine Emissions Characteristics
Fuels consist of Hydrogen (H) and Carbon (C)
molecules. During the combustion period in the
engine cylinder, these C and H molecules react with
oxygen (O2) in the air and converted to the CO, CO2,
HC. These exhaust tail emissions are harmful for
human health and environmental pollution. Carbon
monoxide (CO) is colorless, odorless, tasteless gas
which is lighter than air. It is highly toxic to humans
and animals in higher quantities. CO is a common
industrial hazard resulting from the incomplete
burning of natural gas and any other material
containing carbon.
Figure 5: Influence of n-Butanol gasoline blended fuels on
CO emissions.
The effect of the n-Butanol gasoline blends on CO
emissions for different engine speeds is shown in Fig.
5. It can be seen that when n-Butanol percentage
Investigation of N-Butanol Blending with Gasoline using a 1-D Engine Model
389
increases, the CO concentration decreases. This can
be explained by the enrichment of oxygen owing to
the n-Butanol, in which an increase in the proportion
of oxygen will promote the further oxidation of CO
during the engine exhaust process. Another
significant reason for this reduction is that n-Butanol
(C
4
H
9
OH) has less carbon than gasoline (C
8
H
15
). The
lowest CO emissions are obtained with blended fuel
containing n-Butanol (n-B95).
When using gasoline as fuel in a spark ignition
engine, the unburned fuel hydrocarbons (HC) in the
exhaust consist mainly of unburned gasoline which
itself largely consists of hydrocarbons. However,
when using gasoline-Butanol blends as fuel the un-
combusted fuel constituents include both unburned
gasoline (which consists mainly of hydrocarbons as
noted) and un-combusted Butanol. Thus, the HC
emissions measured in the diluted exhaust consist of
both hydrocarbons and Butanol. From a legal
perspective, HC emissions are regulated by law, but
not Butanol emissions. This means that reported HC
emissions from vehicles fueled with alcohol-gasoline
blends are overestimated, due to the contribution of
the alcohol contents in the exhaust emitted from the
vehicle, and the larger the alcohol contents present in
the exhaust, the greater the error in estimated HC
emissions (Egeback et al., 2005).
Figure 6: Influence of n-Butanol gasoline blended fuels on
HC emissions.
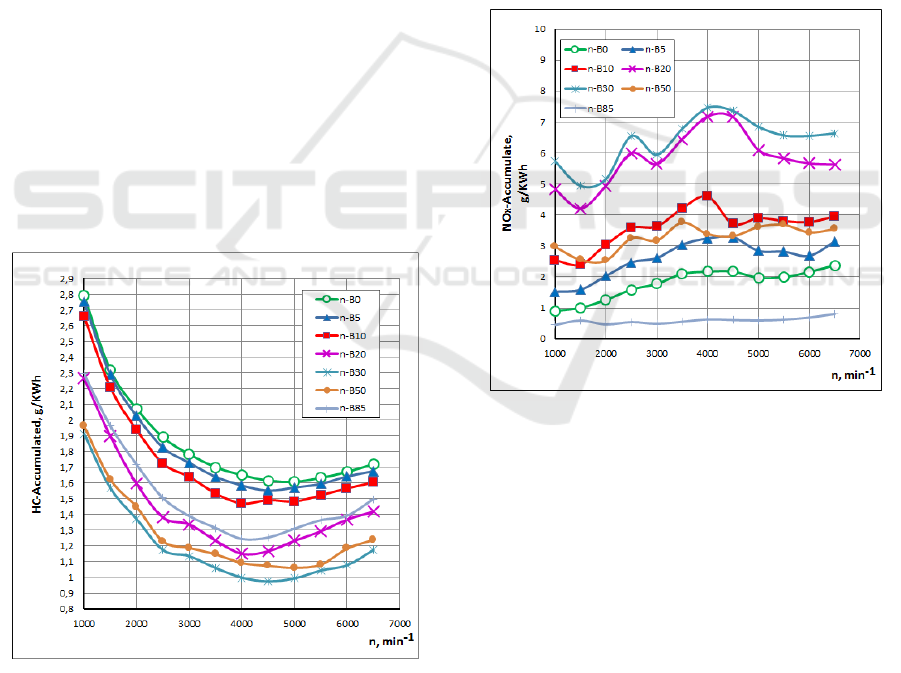
The effect of the n-Butanol gasoline blends on HC
emissions for different engine speeds is shown in Fig.
6. It can be seen that when n-Butanol percentage
increases, the HC concentration decreases. The
concentration of HC emissions decreases with the
increase of the relative air-fuel ratio. The reason for
the decrease of HC concentration is similar to that of
CO concentration described above.
Nitrogen oxides (NO and NO
2
) are formed by the
oxidation of nitrogen from the air in the combustion
process. An important parameter for the formation of
nitrogen oxides is the combustion temperature
(increased combustion temperature results in
increased nitrogen oxide emissions). Therefore, its
probable formation is in very high temperature
regions, which are related to heat release (Raslavicius
L. 2010). It should be noted that nitrogen oxides
(NO
X
) are regulated pollutants that are determined
jointly, as the sum of NO and NO
2
contents rather
than as individual components (Egeback et al., 2005).
Figure 7: Influence of n-Butanol gasoline blended fuels on
NOx emissions.
The effect of the n-Butanol gasoline blends on
NOx emissions for different engine speeds is shown
in Fig. 7. It can be seen that when n-Butanol
percentage increases up to 50% n-B50, the NOx
concentration increase after which it decreased with
increasing n-Butanol percentage. This can be
explained by that improved combustion inside the
cylinder resulting in an increased in-cylinder
temperature. The higher percentage of n-Butanol in
gasoline reduces the in-cylinder temperature. The
reasons for the reduction in temperature are: 1. Latent
heat of evaporation of n-Butanol, which decreases the
in-cylinder temperature when they vaporizes, 2.
There are more triatomic molecules are produced, the
SMS 2017 - Special Session on Sustainable mobility solutions: vehicle and traffic simulation, on-road trials and EV charging
390

higher the gas heat capacity and the lower the
combustion gas temperature will be. However the low
in-cylinder temperature can also lead to an increment
in the unburned combustion product.
4 CONCLUSIONS
The present paper demonstrates the influences of n-
Butanol addition to gasoline on SI engine
performance and emission characteristics. General
results concluded from this study can be summarized
as follows:
- When the n-Butanol content in the blended fuel
was increased, the engine brake power decreased for
all engine speeds. The engine performance of blends
is lower than gasoline due to the combustion
characteristics of n-butanol (higher latent heat and
lower calorific value than gasoline). The lower
saturation pressure of n-butanol compared to gasoline
leads to a lower volumetric efficiency for blended
fuels. The engine performance of blends could be
improved by modifying ignition time and increasing
compression ratio since n-butanol has more resistance
to detonation than gasoline.
- The BSFC increased as the butanol percentage
increased. Also, a slight difference exists between the
BSFC when using gasoline and when using gasoline
blended fuels n-B5, n-B10, n-B20 and n-B30.
- When n-Butanol percentage increases, the CO
and HC concentration decreases.
- Butanol gasoline blends the significant increase
NOx emissions with the increase of butanol
percentage. When butanol percentage increases up to
50% n-B50, the NOx concentration increase after
which it decreased with increasing butanol
percentage.
ACKNOWLEDGEMENTS
We are eternally grateful to AVL-AST, Graz, Austria
for granting use of AVL-BOOST under the university
partnership program.
REFERENCES
Szwajaa, S., Naber, J.D., 2010. Combustion of n-Butanol in
a Spark Ignition IC Engine, Fuel, Vol. 89, Isuse 7,
pp.1573-1582.
Yacoub, Y., Bara, R., Gautam, M., 1998. The performance
and emission characteristics of C1–C5 alcohol–
gasoline blends with matched oxygen content in a
singlecylinder spark ignition engine. Proc Inst Mech
Eng A – Power Energy; 212:363–79.
Gautam, M., Martin, DW., 2000. Combustion
characteristics of higher-alcohol/gasoline blends. Proc
Inst Mech Eng; 214A:497–511.
Alasfour F.N., 1998. NOx emission from a spark-ignition
engine using 30% iso-butanol - gasoline blend: Part 1:
Preheating inlet air, Appl. Therm. Eng. 18, 5, p. 245-56.
Minter, S., 2006. Alcoholic Fuels, Taylor&Francis Group,
270 pages.
Wallner T, Miers SA, McConnell S., 2009. A comparison
of ethanol and butanol as oxygenates using a direct-
injection, spark-ignition engine. J Eng Gas Turbines
Power; 032802:9.
Rakopoulos DC, Rakopoulos CD, Papagiannakis RG,
Kyritsis DC, 2011. Combustion heat release analysis of
ethanol or n-butanol diesel fuel blends in heavy-duty DI
diesel engine. Fuel; 90:1855–67.
Sarathy SM, Thomson MJ, Togbé C, Dagaut P, Halter F,
Mounaim-Rousselle C, 2009. An experimental and
kinetic modeling study of n-butanol combustion.
Combust Flame; 156:852–64.
Dernotte J, Mounaim-Rousselle C, Halter F, Seers P., 2010.
Evaluation of butanol–gasoline blends in a port fuel-
injection, spark-ignition engine. Oil Gas Sci Technol;
65:345–51.
Gu X, Huang Z, Cai J, Gong J, Wu X, Lee CF., 2012.
Emission characteristics of a sparkignition engine
fuelled with gasoline-n-butanol blends in combination
with EGR. Fuel;93:611–7.
Feng R, Yang J, Zhang D, Deng B, Fu J, Liu J, et al., 2013.
Experimental study on SI engine fuelled with butanol-
gasoline blend and H2O addition. Energy Convers
Manage; 74:192–200.
Emilio N, Teresa J, Roberto C, 2013. CO
2
emissions from
a spark ignition engine operation on natural gas-
hydrogen blends (HCNG). Appl Energy;101: 112e20.
Ritchie D, Guohong T, Hongming X, Shijin S, 2012.
Ignition timing sensitivities of oxygenated biofuels
compared to gasoline in a direct-injection SI engine.
Fuel;99:72e82.
S. Iliev, 2014. Developing of a 1-D combustion model and
study of engine characteristics using ethanol-gasoline
blends. Proceedings of the World Congress on
Engineers, Vol II, WCE 2014/ 978-988-19253-5-0.
S. Iliev, 2015. A comparison of ethanol and methanol
blending with gasoline using a 1-D engine model.
Procedia Engineering, Elsevier, Vol 100, pp. 1013-
1022.
L. Raslavicius, Z. Bazaras, 2010. Variations in oxygenated
blend composition to meet energy and combustion
characteristics very similar to the diesel fuel, Fuel
Processing Technology 91 (9) 1049–1054.
Egeback KE, Henke M, Rehnlund B, Wallin M,
Westerholm R, 2005. Blending of ethanol in gasoline
for spark ignition engines-problem inventory and
evaporative measurements. Report No. MTC 5407.
AVL MTC Motortestcenter AB Box 223 SE-136 23
Haninge.
Investigation of N-Butanol Blending with Gasoline using a 1-D Engine Model
391
