
The Cytotoxicity of Paclitaxel Was Smaller than Doxorubicin
in T47D Breast Cancer Cell
Irma Yanti Rangkuti
Biomedic/Pharmacology Department, Faculty of Medicine, Universitas Islam Sumatera Utara, Indonesia
Keywords: Paclitaxel, doxorubicin, cytotoxicity, T47D breast cancer cell.
Abstract: Breast cancer is a type of cancer commonly found by most women, ranking fifth leading cause of cancer
worldwide cancer, consist of various subtypes. Chemotherapy is one of therapy in breast cancer patients but
the benefit is not equal for all patients and paclitaxel is one of the most important chemotherapeutic drugs.
The descriptive study had been done to paclitaxel and doxorubicin in T47D breast cancer cell. This study
aimed to compare cytotoxic effect of paclitaxel and doxorubicin in T47D breast cancer cell, used paclitaxel
concentrations 1000; 500; 250; 31,25; 15,625 nM and doxorubicin concentrations 500; 250;62,5;31,25;and
15,625 nM for 24 hours. This study was an invitro. The cytotoxic test used MTT method to determine IC
50
and analized by SPSS. The result showed that IC
50
paclitaxel was 1577,2 ± 115,3 nM and IC
50
doxorubicin
202,37 ± 3,99 nM. The cytotoxicity of paclitaxel was smaller than doxorubicin in T47D breast cancer cell.
1 INTRODUCTION
Breast cancer is a disease in which there is excessive
growth or uncontrolled development of breast tissue
cells. Breast cancer is a type of cancer commonly
found by most women, ranking fifth leading cause of
cancer worldwide cancer around 522,000 deaths and
the most common cause of death in women in
developing countries (324,000 deaths). According to
IARC's Globocan data (International Agency for
Research on Cancer) in 2012 there are 14,067,894
new cases of cancer and 8,201,575 deaths from
cancer worldwide with the most cancer types of
breast cancer, prostate cancer and lung cancer.
Estimated 1.67 million new cases breast cancer in
2012. For Indonesia, the incidence of cancer in
women is about 134 per 100,000 population with
most cases of breast cancer of 40 per 100,000
women. Globocan estimates, deaths in Indonesia due
to breast cancer approximately 16.6 deaths per
100,000 population (Kemenkes RI, 2016).
Breast cancer consist of subtypes based on IHC
markers including ER, progesterone receptor (PR)
and human epidermal growth factor receptor 2
(HER2). Breast tumors are grouped into four basic
subgroups according to these markers, i.e.,
[ER+|PR+]HER2 - (tumors with either ER or PR
positivity, and HER2 negativity), [ER+|PR+]HER2+
(tumors with either ER or PR positivity, and HER2
positivity), ER –PR -HER2+ (tumors with ER and
PR negativity, and HER2 positivity, also named
HER2 positive), ER –PR -HER2 - (tumors with ER,
PR, HER2 negativity, also named triple negative)
(Dai et al. 2016).
Chemotherapy is one of therapy in breast cancer
patients and improves survival of patients with stage
I–III breast cancer, but the benefit is not equal for
all patients because there are melocular
characteristics of the cancer affect sensitivity to
chemotherapy (Andre and Pusztai 2006; Hassan et al.
2010). The use of cytotoxic chemotherapy in both
advanced and early stage breast cancer has made
significant progress in the last 10 years with several
landmark studies identifying clear survival benefits
for newer therapies (Hassan et al. 2010).
Paclitaxel is one of the most important
chemotherapeutic drug, isolated from the Pacific
yew, Taxusbrevifolia, was approved for the
treatment of metastatic breast cancer in 1994 (Patt,
Gauthier, and Giordano 2006). This drug causes
abnormal stabilization of the dynamic microtubule
polymerization that alters intracellular signaling,
transport organelle and locomotion, and leading to
the failure of mitosis (Honore, Pasquier, and Braguer
2005). Recent studies showed that paclitaxel is able
to induce reactive oxygen species (ROS) production
in cancer cells and hydrogen peroxide (H2O2) that
Rangkuti, I.
The Cytotoxicity of Paclitaxel Was Smaller than Doxorubicin in T47D Breast Cancer Cell.
DOI: 10.5220/0010061801710176
In Proceedings of the 7th International Conference on Multidisciplinary Research (ICMR 2018) - , pages 171-176
ISBN: 978-989-758-437-4
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
171

involved in cancer cell death with Paclitaxel
(Alexandre, Batteux, et al. 2006; Ramanathan et al.
2005; Alexandre, Nicco, et al. 2006). The novel
mechanism of paclitaxel by inducing toxic bystander
effect through generation of extracellular H2O2
from the membrane–associated NOX (Alexandre et
al. 2007).
Doxorubicin is considered to be the most
effective agent in the treatment of breast cancer
patients (Smith et al. 2006). Doxorubicin is an
anthracyline drug first extracted from Streptomyces
peucetius var. caesius in the 1970’s. The
mechanisms of action doxorubicin in cell are
intercalation into DNA and disruption of
topoisomerase-II-mediated DNA repair and
generation of free radicals and their damage to
cellular membranes, DNA and proteins. Doxorubicin
is oxidized to semiquinone, an unstable metabolite,
which is converted back to doxorubicin in a process
that releases reactive oxygen species. Reactive
oxygen species can lead to lipid peroxidation and
membrane damage, DNA damage, oxidative stress,
and triggers apoptotic pathways of cell death (Thorn
et al. 2011).
The T47D cell is one of breast cancer cell that
used for research. The characteristic this cell is a
continuous cell line isolated from a breast ductal
tumor tissue of a 54-year-old woman who expresses
a mutated p53 protein (missense mutation) at a 194
residue (in zinc-binding domain, L2).
2 MATERIALS AND METHODS
This study used paclitaxel and doxorubicin as
materials. Paclitaxel was obtained from PT Dankos
Farma, Indonesia.
This research was an invitro, descriptive study
to compare cytotoxic effect of paclitaxel and
doxorubicin in T47D breast cancer cell, used
paclitaxel concentrations 1000; 500; 250; 31,25;
15,625 nM and doxorubicin concentrations 500;
250;62,5;31,25; and 15,625 nM for 24 hours. The
cytotoxic test used MTT [3-(4,5-dimetiltiazol-2-il)-
2,5-difenil tetrazolium bromida] method to
determine IC
50
and analized by SPSS.
2.1 Cell Culture
In this study, T47D cells obtained from the
Laboratory of Parasitology, Faculty of Medicine,
Gadjah Mada University were grown in RPMI
medium containing 10% Fetal Bovine Serum (Gibco,
USA), 2% Penicillin-Streptomycin (Gibco, USA),
and Fungizone (Amphotericin B) 0.5% (Gibco, USA)
on the flask in a humidified incubator(5% CO
2
/95%
air) at 37°C (Doyle and Bryan, 1998).
2.2 Cell Viability Assay
The viability of T47D cells was assessed using the
MTT assay. The cells were cultivated on 96 well
plates (Iwaki, Japan). Each well contains 1x10
4
cells.
The cells incubated in a humidified incubator
(5% CO
2
/95% air) for 24 hours. After 24 hours
incubation, the medium culture is discharged and
each well is given paclitaxel with concentration1000;
500; 250; 31,25; 15,625 nM. After 24 hours
incubation, the cells was incubated with 0.5 mg/mL
MTT (Sigma-Aldrich, USA) for 4 hours at 37°C.
The cells that is feasible to react with MTT to
produce of purple crystals formazan. After 4 hours,
10% SDS (Sigma-Aldrich, USA) stopper in 0.01 N
HCl (Merck, USA) was added to dissolve the
formazan crystals. Then, the cells are incubated for
24 hours at room temperature and protected from
light. After incubation, cells were shaken, and cell
absorbance was measured by elisamicroplate reader
(Bio-Rad, USA) at λ 595 nm. The experimental data
were the absorbance of each well, and then
converted to percentage of a cells viable using
equation as indicated below
% of viable cells =
B
–
C
A – C
x 100%
(1)
Where A, B, and C (1) respectively are absorbance
of control cells absorbance, treated cells absorbance,
and medium culture absorbance. All data were
expressed as IC
50
that calculate using probate
regression analysis at SPSS, test were used for
statistical analyses with p values <0.05 were
considered significant (Meiyanto et al. 2008).
3 RESULT AND DISCUSSION
Cytotoxic assay is a preliminary test to determine
the potential toxicity of a compound and IC
50
as a
mainly parameters. T47D cells were exposed to
paclitaxel using concentration series of 1000; 500;
250; 31,25; 15,625 nM for 24 hours. After analyzed,
IC
50
paclitaxel = 1577,2 ± 115,3 nM.
The result of cytotoxic test paclitaxel against
T47D cells during 24 hours exposure can be seen in
Figure 1.
ICMR 2018 - International Conference on Multidisciplinary Research
172
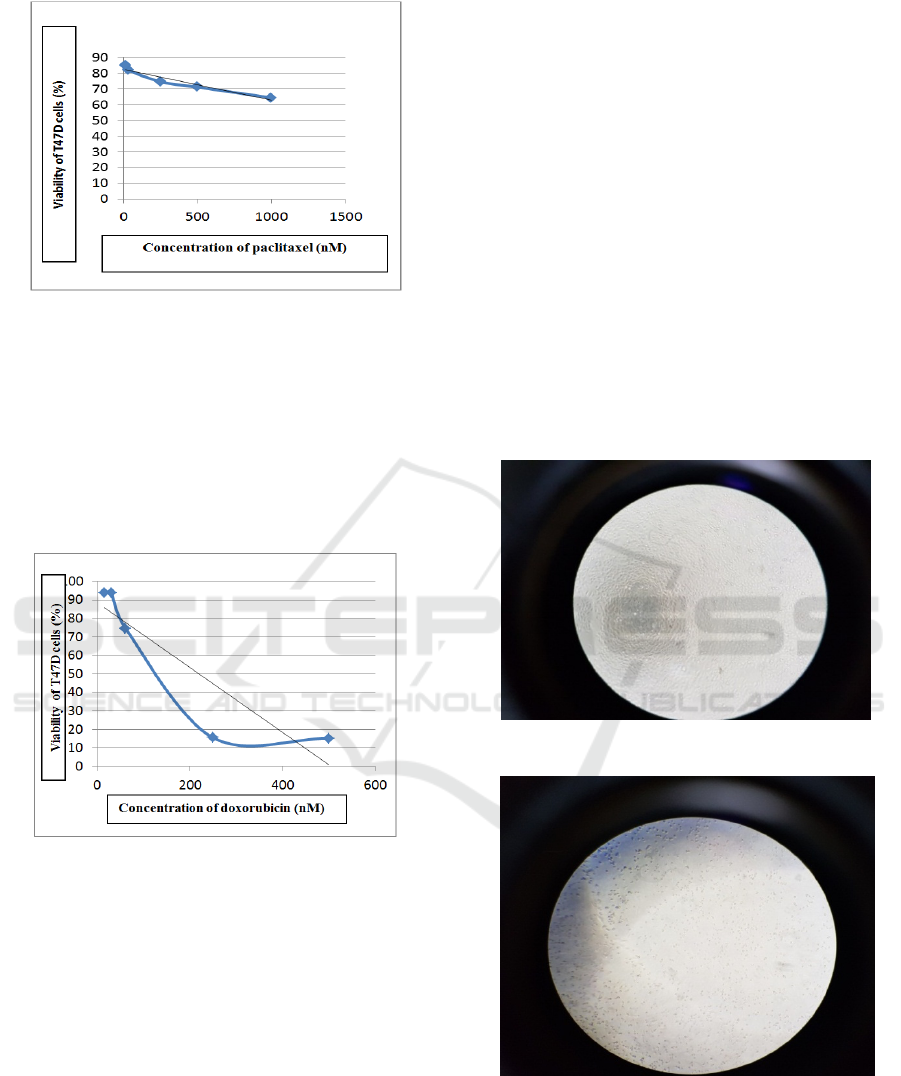
Figure 1: Graph the effect of paclitaxel concentration on
T47D cell viability.
In the cytotoxic paclitaxel test, doxorubicin was
used as a positive control, one of the chemotherapy
for breast cancer using concentration series of 500;
250;62,5;31,25 and 15,625 nM. After analysis, IC
50
doxorubicin = 202,37 ± 3,99 nM. The result of
doxorubicin cytotoxic test can be seen in Figure 2
below.
Figure 2: Graph the effect of doxorubicin concentration on
viability T47D cell.
This study used the MTT assay to test
cytotoxicity of drug, a quantitative method was
measured by elisamicroplate reader (Bio-Rad, USA)
at λ 595 nm. Cytotoxic effects are indicated by IC
50
values, the concentration that causes death in 50% of
the cell population by calculating living cells.
The principle of MTT assay is colorimetry
(measurement of color intensity) based on the
formation of formazan crystals (purple and
filamentous) in living cells, penetrating the
membrane and accumulating in them (Figure 3 - 8).
Color formation in living cells as a result of
metabolizing a substrate by living cells into colored
products. The tetrazolium succinate reductase
system found in the living cell mitochondria
included in the respiratory chain will reduce the
MTT yellow to form purple formazan crystals (van
Meerloo, Kaspers, and Cloos, 2011).
Dead cells cannot form formazan crystals
because dead cells are unable to aspire so that
tetrazolium succinate enzymes which can reduce
MTT salt to formazan products are not produced, so
the color of dead cells is not purple but will remain
yellow. The more cells that live, the purple will
become thicker (Freshney, 2015). These figures
below were T47D breast cancer cell looked by
microscope.
A. T47D breast cancer cell before MTT
These figures below showed T47D cell before
MTT, consist of control cell, T47D with
doxorubicin exposure and paclitaxel (Figure 3
– 5).
Figure 3: Control cell (T47D without drug).
Figure 4: T47D cell after giving doxorubicin 500 nM for
24 hours.
The Cytotoxicity of Paclitaxel Was Smaller than Doxorubicin in T47D Breast Cancer Cell
173
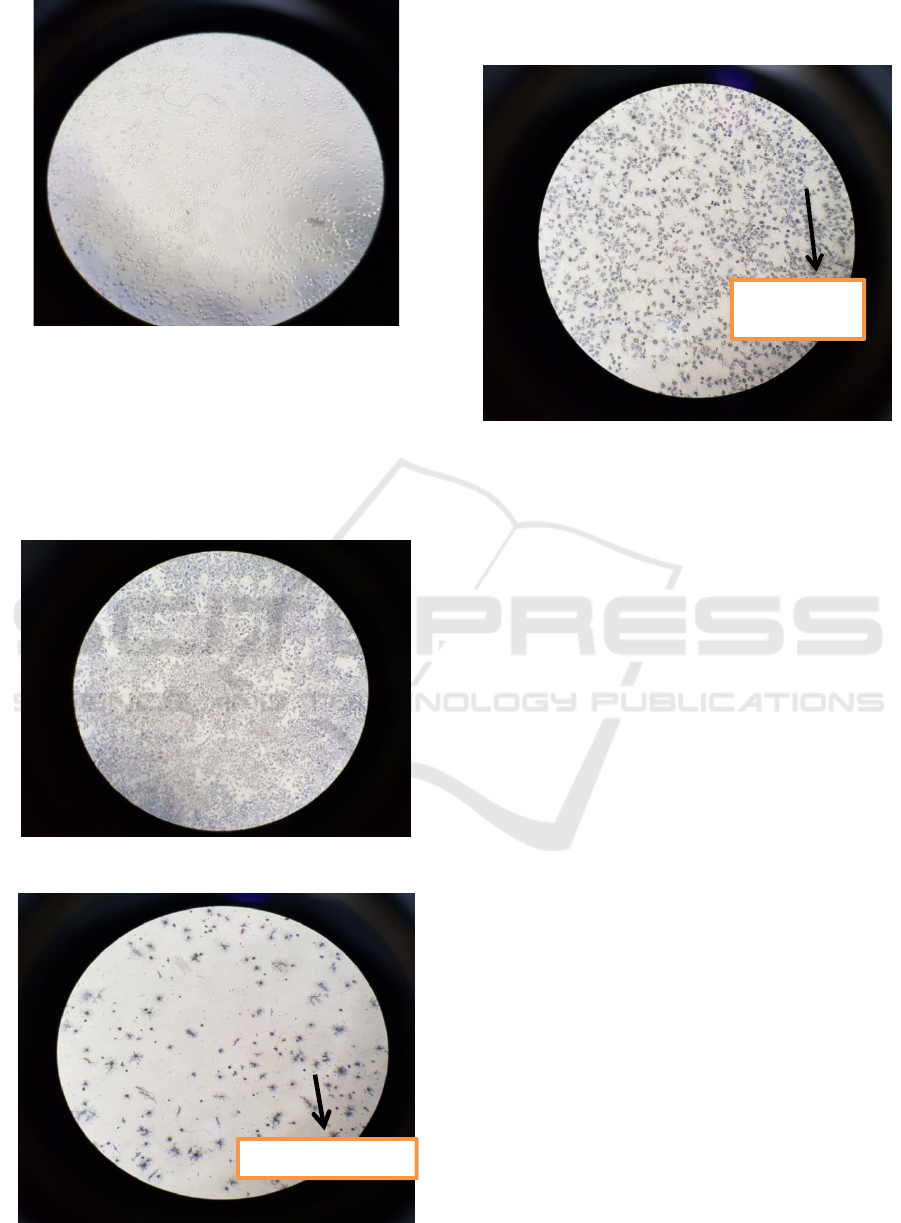
Figure 5: T47D cell after giving paclitaxel 1000 nM for 24
hours.
B. T47D breast cancel after MTT
These figures below showed T47D cell after
MTT, consist of control cell, T47D with
doxorubicin exposure and paclitaxel (Figure 6
– 8).
Figure 6: Control cell (T47D without drug).
Figure 7: T47D cell after giving doxorubicin 500 nM for
24 hours (= formazan crystal).
Figure 8: T47D cell after giving paclitaxel 1000 nM for 24
hours (= formazan crystals).
Based on IC
50,
from cytotoxic test, it showed that
cytotoxicity of paclitaxel was smaller than
doxorubicin. Study in 2012 found that paclitaxel was
resistant in T47D breast cancer cell associated with
highly expresses Lin28 in T47D cells than the
MCF7, Bcap-37 or SK-BR-3 cancer cell lines, which
had low-level expression of Lin28. This study
knocked down of Lin28 in Lin28 high expression
T47D cells, the result showed increasing the
sensitivity to paclitaxel treatment, while stable
expression of Lin28 in breast cancer cells effectively
attenuated the sensitivity to paclitaxel treatment,
resulting in a significant increase of IC
50
values of
paclitaxel. Transfection with Lin28 also significantly
inhibited paclitaxel-induced apoptosis. This study
showed that Lin28 expression was dramatically
increased in tumor tissues after neoadjuvant
chemotherapy or in local relapse or metastatic breast
cancer tissues. Moreover, further studies showed
that p21, Rb and Let-7 miRNA were the molecular
targets of Lin28. Overexpression of Lin28 in breast
cancer cells considerably induced p21 and Rb
expression and inhibited Let-7 miRNA levels (Lv et
al. 2012).
p21, universal inhibitor of cyclin kinases inhibits
the activity of each member of the cyclin/CDK
family (Xiong et al. 1993), promote cell cycle arrest
in cell cycle (Karimian, Ahmadi, and Yousefi, 2016).
Cell cycle is a cell proliferation process that
mediates the growth and development of living
things (Nurse, 2000). The Rb protein is a tumor
suppressor, which plays a pivotal role in the negative
control of the cell cycle and in tumor progression. It
Formazan crystal
Formazan
cristals
ICMR 2018 - International Conference on Multidisciplinary Research
174

has been shown that Rb protein (pRb )is responsible
for a major G1 checkpoint, blocking S-phase entry
and cell growth (Giacinti and Giordano, 2006;
Foster et al. 2001).
Doxorubicin is an anthracycline breast cancer
drug that is still used in combination regimens and
also for other types of cancer such as leukemia
(Wattanapitayakul et al. 2005). Doxorubicin was
used as a positive control in this study, because this
drug is still used, but also doxorubicin showed an
anticancer effect on T47D cells (Barzegar et al.
2015). Cytotoxic activity of doxorubicin through
topoisomerase II inhibition, DNA intercalation, cell
membrane binding and semiquinone free radical
formation and oxygen free radicals (Bruton et al,
2005). Doxorubicin causes the activation of various
molecular signals from AMPK (AMP‐activated
protein kinase inducing apoptosis) to influence the
Bcl‐2/Bax apoptosis pathway. By altering the Bcl‐
2/Bax ratio, downstream activation of different
caspases can occur resulting in apoptosis (Tacar,
Sriamornsak, and Dass, 2013)
4 CONCLUSION
IC
50
paclitaxel was 1577,2 ± 115,3 nM and IC
50
doxorubicin 202,37 ± 3,99 nM. The cytotoxicity of
paclitaxel was smaller than doxorubicin in T47D
breast cancer cell.
REFERENCES
Alexandre, Jérôme, Frédéric Batteux, Carole Nicco,
Christiane Chéreau, Alexis Laurent, Loïc Guillevin,
Bernard Weill, and François Goldwasser. 2006.
'Accumulation of hydrogen peroxide is an early and
crucial step for paclitaxelinduced cancer cell death
both in vitro and in vivo', International journal of
cancer, 119: 41-48.
Alexandre, Jérôme, Yumin Hu, Weiqin Lu, Helene
Pelicano, and Peng Huang. 2007. 'Novel action of
paclitaxel against cancer cells: bystander effect
mediated by reactive oxygen species', Cancer research,
67: 3512-17.
Alexandre, Jérôme, Carole Nicco, Christiane Chéreau,
Alexis Laurent, Bernard Weill, François Goldwasser,
and Frédéric Batteux. 2006. 'Improvement of the
therapeutic index of anticancer drugs by the
superoxide dismutase mimic mangafodipir', Journal of
the National Cancer Institute, 98: 236-44.
Andre, Fabrice, and Lajos Pusztai. 2006. 'Molecular
classification of breast cancer: implications for
selection of adjuvant chemotherapy', Nature Clinical
Practice Oncology, 3: 621.
Barzegar, Elmira, Shamileh Fouladdel, Tahereh Komeili
Movahhed, Shekoufeh Atashpour, Mohammad
Hossein Ghahremani, Seyed Nasser Ostad, and
Ebrahim Azizi. 2015. 'Effects of berberine on
proliferation, cell cycle distribution and apoptosis of
human breast cancer T47D and MCF7 cell lines',
Iranian journal of basic medical sciences, 18: 334.
Dai, Xiaofeng, Liangjian Xiang, Ting Li, and Zhonghu
Bai. 2016. 'Cancer hallmarks, biomarkers and breast
cancer molecular subtypes', Journal of Cancer, 7:
1281.
Doyle, Alan, and Griffins J Bryan. 1998. Cell and Tissue
Culture: Laboratory Procedure in Biotechnology
(John Willey & Sons: Chicester).
Foster, James S, Donald C Henley, Shamila Ahamed, and
Jay Wimalasena. 2001. 'Estrogens and cell-cycle
regulation in breast cancer', Trends in Endocrinology
& Metabolism, 12: 320-27.
Freshney, R Ian. 2015. Culture of animal cells: a manual
of basic technique and specialized applications (John
Wiley & Sons).
Giacinti, C, and A Giordano. 2006. 'RB and cell cycle
progression', Oncogene, 25: 5220.
Hassan, MSU, J Ansari, D Spooner, and SA Hussain. 2010.
'Chemotherapy for breast cancer', Oncology reports,
24: 1121-31.
Honore, S, E Pasquier, and D Braguer. 2005.
'Understanding microtubule dynamics for improved
cancer therapy', Cellular and Molecular Life Sciences
CMLS, 62: 3039-56.
Karimian, Ansar, Yasin Ahmadi, and Bahman Yousefi.
2016. 'Multiple functions of p21 in cell cycle,
apoptosis and transcriptional regulation after DNA
damage', DNA repair, 42: 63-71.
Kemenkes RI. 2016. Stop Kanker. Jakarta: Pusat Data dan
Informasi Kesehatan Republik Indonesia.
Lv, Kezhen, Liqun Liu, Linbo Wang, Jiren Yu, Xiaojiao
Liu, Yongxia Cheng, Minjun Dong, Rongyue Teng,
Linjiao Wu, and Peifen Fu. 2012. 'Lin28 mediates
paclitaxel resistance by modulating p21, Rb and Let-
7a miRNA in breast cancer cells', PloS one
, 7: e40008.
Meiyanto, Edy, Ratna Asmah Susidarti, Sri Handayani,
and Fitria Rahmi. 2008. 'Ekstrak Etanolik Biji Buah
Pinang (Areca catechu L.) mampu menghambat
proliferasi dan memacu apoptosis sel MCF-7',
Majalah Farmasi Indonesia, 19: 12-19.
Nurse, Paul. 2000. 'A long twentieth century of the cell
cycle and beyond', Cell, 100: 71-78.
Patt, Debra, Michelle Gauthier, and Sharon Giordano.
2006. 'Paclitaxel in breast cancer', Women’s Health, 2:
11-21.
Ramanathan, Balakrishnan, Kun-Yan Jan, Chien-Hung
Chen, Tzyh-Chyuan Hour, Hong-Jen Yu, and Yeong-
Shiau Pu. 2005. 'Resistance to paclitaxel is
proportional to cellular total antioxidant capacity',
Cancer research, 65: 8455-60.
Smith, Laura, Mark B Watson, Sara L O'Kane, Philip J
Drew, Michael J Lind, and Lynn Cawkwell. 2006.
'The analysis of doxorubicin resistance in human
The Cytotoxicity of Paclitaxel Was Smaller than Doxorubicin in T47D Breast Cancer Cell
175

breast cancer cells using antibody microarrays',
Molecular cancer therapeutics, 5: 2115-20.
Tacar, Oktay, Pornsak Sriamornsak, and Crispin R Dass.
2013. 'Doxorubicin: an update on anticancer molecular
action, toxicity and novel drug delivery systems',
Journal of Pharmacy and Pharmacology, 65: 157-70.
Thorn, Caroline F, Connie Oshiro, Sharon Marsh, Tina
Hernandez-Boussard, Howard McLeod, Teri E Klein,
and Russ B Altman. 2011. 'Doxorubicin pathways:
pharmacodynamics and adverse effects',
Pharmacogenetics and genomics, 21: 440.
van Meerloo, Johan, Gertjan JL Kaspers, and Jacqueline
Cloos. 2011. 'Cell sensitivity assays: the MTT assay.'
in, Cancer cell culture (Springer).
Wattanapitayakul, Suvara K, Linda Chularojmontri,
Angkana Herunsalee, Suphan Charuchongkolwongse,
Somchit Niumsakul, and John A Bauer. 2005.
'Screening of antioxidants from medicinal plants for
cardioprotective effect against doxorubicin toxicity',
Basic & clinical pharmacology & toxicology, 96: 80-
87.
Xiong, Yue, Gregory J Hannon, Hui Zhang, David Casso,
Ryuji Kobayashi, and David Beach. 1993. 'p21 is a
universal inhibitor of cyclin kinases', nature, 366.
ICMR 2018 - International Conference on Multidisciplinary Research
176
