
Trichoderma asperellum Cell Density in Several Carriers
Risna Maya Sari
1
, Tengku Sabrina
1
, and Mukhlis
1
1
Department of Agriculture, Universitas Sumatera Utara, Medan, Indonesia Republic-20155
Keywords: Biochar, Carrier materials, Cell density, Storage period, Trichoderma asperellum.
Abstract: Biofertilizers was a carrier-based inoculant containing latent microorganisms. The incorporation of
microorganisms in the carrier allows easy long-term storage and high effectiveness of biofertilizers. The
aims of this study were to obtain the best carrier in maintaining population of Trichoderma asperellum. The
research used completely randomized design, consist of six carrier material. The materials tested for its
ability as a carrier were the mixed of empty fruit bunches compost + azolla + chicken egg shell + poultry
manure; the mixed of rice straw + azolla + chicken egg shell + poultry manure; the mixed of rice husk +
azolla + chicken egg shell + poultry manure; empty fruit bunches biochar; rice husk biochar and cow bone
biochar. Research was conducted in soil biology laboratory, Agriculture, Universitas Sumatra Utara, Medan
from April 2018 until May 2018.The result showed the cell density of T. asperellum after 8 weeks of
storage on all carrier treatments was increasing steadily. The carriers with the highest population T.
asperellum after 8 weeks of storage was the mixed of rice straw + azolla + chicken egg shell + poultry
manure. The carbon and nitrogen content of the carrier affects the cells density of T. asperellum.
1 INTRODUCTION
Fertilizer is a material that is given into the soil to
fulfill the requirement nutrients for plants to growth
and production. Inorganic fertilizer is the choice of
farmers to meet the nutrient needs of the plant. Since
the green revolution, fertilization is doing
intensively in the most agricultural system.
However, unfortunately the productivity of the
plants is not significant increase economically now
(Parman, 2007).
Biological fertilizer (biofertilizer) is an
alternative way to reduce dependence on inorganic
fertilizers. Biological fertilizer made from carrier
containing living cells or microbes (Rao, 1982).
Inoculated microbes into biological fertilizers can
serve as nutrient providers as well as a remodel of
soil organic matter. Biological fertilizers have been
shown to increase the ability of plant nutrient
uptake, to accelerate composting process, and to
improve soil structure (Tombe, 2008).
Biological fertilizers are generally packed in the
liquid form. The formulation of biofertilizer in liquid
form has several drawbacks, like: the difficulty in
packaging, distribution, application, storage, and
quality of fertilizer will be reduced if stored in the
longer period. The quality of the fertilizer is reduced
due to the decline of microbial population and the
low resistance of microbes contained in the
biological fertilizer. Carrier is considered as the best
solution to overcome the deficiency (Putri, 2010).
Combination materials formed carrier is a new
alternative medium that can be used to grow, pack,
and extend microbial storage time. The carrier
composition must contain important components
(organic nutrients) that can support microbial growth
(Firdausi, 2016).
One type of microbe often inoculated into the
biological fertilizer is Trichoderma sp. because
Trichoderma sp. can grow in various propagation
media. Trichoderma sp. is one of the soil
saprophytic fungi which is advantageous to the plant
due to its antagonistic properties with the pathogen
(Gusnawaty, 2017). Trichoderma sp. can also be
used to accelerate the decomposition process of
organic materials such as carbohydrates (cellulose)
with the help of cellulose enzyme (Rinata, 2016).
Microbe carrier medium used in this study was
avoided from materials that can be used as animal
feed; as is usually done inoculum local seller are
using bran, corn flour or rice.
20
Sari, R., Sabrina, T. and Mukhlis, .
Trichoderma asperellum Cell Density in Several Carriers.
DOI: 10.5220/0010073800200025
In Proceedings of the International Conference of Science, Technology, Engineering, Environmental and Ramification Researches (ICOSTEERR 2018) - Research in Industry 4.0, pages
20-25
ISBN: 978-989-758-449-7
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

2 MATERIALS AND METHODS
This research was conducted at Soil Biology
Laboratory, Agriculture, Universitas Sumatra Utara,
Medan on April 2018 until May 2018.
The materials used as carrier of T. asperellum
were empty fruit bunches compost, rice straw, rice
husk, azolla, chicken egg shell, poultry manure,
biochar of empty fruit bunches, biochar of rice husk,
biochar of cow bone.
This study was used: completely randomized
design, consisting of six treatments and repeated
four times. Treatmentswere:
A : Mixture of empty fruit bunches compost
(70%) + azolla (10%) + chicken egg
shell (10%) + poultry manure (10%)
B : Mixture of rice straw (70%) + azolla
(10%) + chicken egg shell (10%) +
poultry manure (10%)
C : Mixture of rice husk (70%) + azolla
(10%) + chicken egg shell (10%) +
poultry manure (10%)
D : Biochar of empty fruit bunches (100%)
E : Biochar of rice husk (100%)
F : Biochar of cow bone (100%)
2.1 Preparation of Medium Carrier
Carrier materials were milled to pass through <2 mm
mesh sieved, then mixed according to the
composition of each treatment. Each 10 g carrier
were packed in an autoclaveable plastic bag. Then
all the carrier medium was sterilized using an
autoclave for two consecutive days (121
o
C and 1
atm pressure for 15 minutes). After completion of
the autoclave process on the second day, water vapor
in the plastic was allowed to dry first, then the
plastic containing the carrier material sealed tightly
using a sealer.
The carbon, nitrogen, phosphate and potassium
content of each carrier medium were analyzed
(Table 1).
2.2 Preparation of T.asperellum
Trichoderma asperellum used was a collection of
soil biological laboratory, Agriculture, Universitas
Sumatra Utara, Medan, that was isolated from the
chipping trunk of oil palm tree (Sabrina, et al.,
2017). Brooth culture of T. asperellum having a cell
density 10
8
cells / mL was used to inoculate. All bags
stored in room temperature (25
o
C).
2.3 Cell Density Observation
Observation of cell density was taken every 2 weeks
during the storage period. Cell density was
calculated using haemocytometer, with the formula
(Gabriel & Riyatno, 1989):
(1)
Description:
K : Number of cells / mL of solution
t : Number of cells in the sample box
observed
n : Number of sample boxes
0.25 : Correction factor for use of small sample
boxes on haemocytometer
3 RESULT AND DISCUSSION
The growth of T. asperellum in different carrier
medium during the 2nd, 4th, 6th, and 8th week
stored time is showed at Table 2.
Table 1: Carbon, Nitrogen, Phosphate and Potassium Analysis of Carrier Medium
Carrier
Water content (%)
C-organic (%)
Nitrogen (%)
Phosphate (%)
Potassium (%)
A
18.81
18.78
1.92
0.27
0.35
B
17.27
27.95
2.26
0.30
1.12
C
9.56
33.85
0.89
0.24
0.55
D
7.48
1.45
0.07
0.66
49.70
E
51.67
6.20
0.72
0.13
0.54
F
13.74
4.96
1.88
0.83
0.04
Trichoderma asperellum Cell Density in Several Carriers
21
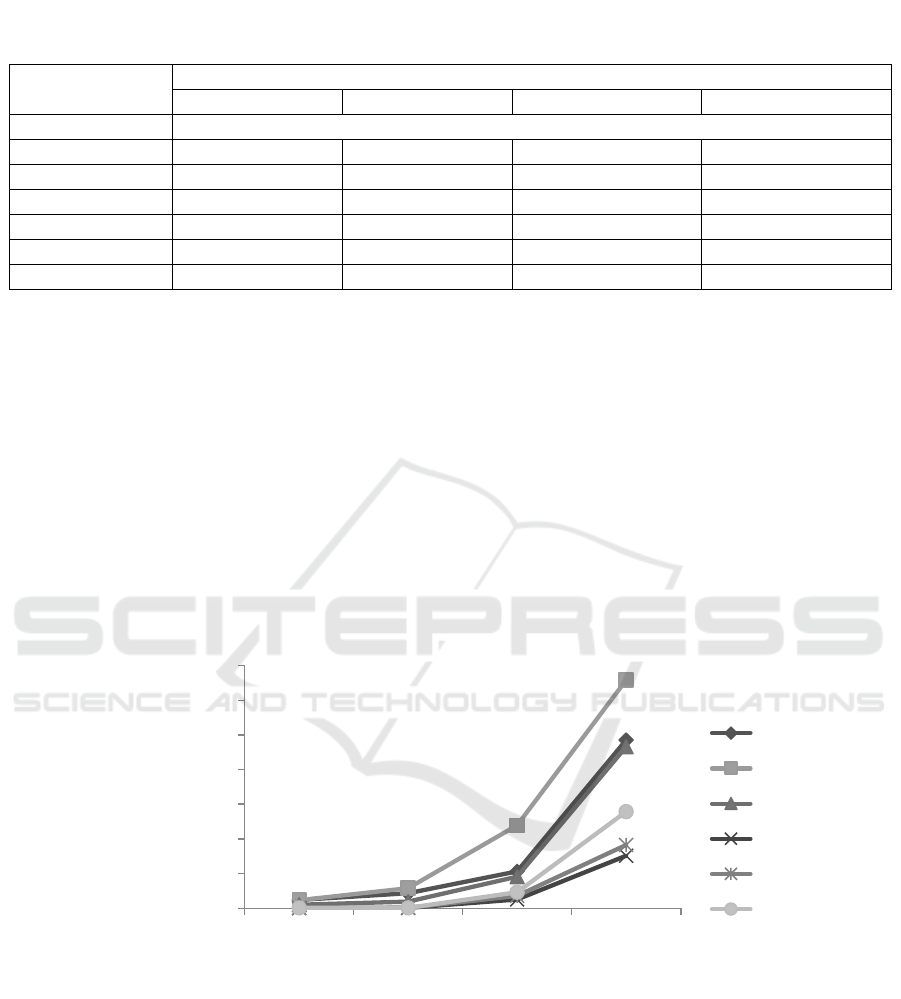
Observation at 2-week storage period showed the
highest cells density of T. asperellum was obtained
from the mixed carrier of empty fruit bunches
compost (70%) + azolla (10%) + chicken egg shell
(10%) + poultry manure (10%) (A). While at 4, 6,
and 8 week storage period, the highest cells density
of T. asperellum was obtained from the mixture
carrier that consist of rice straw (70%) + azolla
(10%) + chicken egg shell (10%) + poultry manure
(10%) (B). The cells density of T. asperellum in all
carrier medium increased during storage time. It
may be due to the carrier contain the suitable organic
materials that can ensure the survival of microbes
during the storage (El-Fattah, et al., 2013).
However, the cell number in the carrier formulated
from the mixture of materials was higher than the
cell density of the carrier formulated from biochar
(Figure 1).
Figure 1: Cell density of Trichoderma asperellum in each carrier materials
0,00
50,00
100,00
150,00
200,00
250,00
300,00
350,00
2 4 6 8
Cell Density (10
7
cells/mL)
Time Observation (week)
Cell Density in Carrier Materials
A
B
C
D
E
F
Table 2: The Cell Density of Trichoderma asperellum in Several Carrier During Storage Period
Carrier
Storage Period (Week)
2
4
6
8
10
7
cells / g carrier
A
12.18 a
21.73 b
53.13 b
242.50 b
B
11.68 b
29.08 a
119.75 a
329.00 a
C
5.29 c
9.79 c
45.63 c
232.75 c
D
0.30 f
0.61 f
12.94 f
75.63 f
E
0.53 e
0.66 e
18.13 e
91.25 e
F
0.62 d
0.71 d
23.00 d
139.38 d
Description: The number followed with different letters in each storage period (same column) are significant
different at 5% level based on LSD test.
ICOSTEERR 2018 - International Conference of Science, Technology, Engineering, Environmental and Ramification Researches
22

Figure 2: Cell density of Trichoderma asperellum during storage period
Increased cell density of T. asperellum in the
carrier medium consist of the mixture of empty fruit
bunches compost (70%) + azolla (10%) + chicken
egg shell (10%) + poultry manure (10%) (A), the
mixed of rice straw (70%) + azolla (10%) + chicken
egg shell (10%) + poultry manure (10%) (B), and
the mixed of rice husk (70%) + azolla (10%) +
chicken egg shell (10%) + poultry manure (10%) (C)
between observations ranged between 4.50 x 10
7
-
209.25 x 10
7
cells/g, while on treatment of the
biochar of empty fruit bunches (100%) (D), the
biochar of rice husk (100%) (E), and the biochar of
cow bone (100%) (F) is ranging from 0.09 x 10
7
-
116.38 x 10
7
cells/g (Table 3). Trichoderma
asperellum is able growth in a variety of habitats and
environments (Prabowo, 2006). Trichoderma
asperellum also has a role as a biodecomposer and
able to utilize organic materials containing cellulose
(Widyastuti, 2001). To accelerate the availability of
nutrients for its growth, T. asperellum produces
cellulase enzymes that can degrade cellulose
(Ratanaphadit, 2010). Increased higher cell density
on carrier from mixture materials (A, B, and C) was
possibly caused by the higher organic carbon (18.78-
33.85%) and nitrogen (0.89-2.26%) content of the
medium (Tabel 1).
12,18
11,68
5,29
0,30
0,53
0,62
0,00 5,00 10,00 15,00
A
B
C
D
E
F
Cell Density (10
7
cells/mL)
Carriers
Cell Density in 2nd week
21,73
29,08
9,79
0,61
0,66
0,71
0,00 10,00 20,00 30,00 40,00
A
B
C
D
E
F
Cell Density (10
7
cells/mL)
Carriers
Cell Density in 4th week
53,13
119,75
45,63
12,94
18,13
23,00
0,00 50,00 100,00 150,00
A
B
C
D
E
F
Cell Density (10
7
cells/mL)
Carriers
Cell Density in 6th week
242,50
329,00
232,75
75,63
91,25
139,38
0,00 100,00 200,00 300,00 400,00
A
B
C
D
E
F
Cell Density (10
7
cells/mL)
Carriers
Cell Density in 8th week
Trichoderma asperellum Cell Density in Several Carriers
23

4 CONCLUSIONS
Cell density T. asperellumin a different carrier
increased to 8 weeks of storage. Cell density on the
treatment of empty fruit bunches compost (70%) +
azolla (10%) + chicken egg shell (10%) + poultry
manure (10%) (A), the mixed of rice straw (70%) +
azolla (10%) + chicken egg shell (10%) + poultry
manure (10%) (B), and the mixed of rice husk (70%)
+ azolla (10%) + chicken egg shell (10%) + poultry
manure (10%) (C) higher than in the treatment of
biochar of empty fruit bunches (100%) (d), biochar
of rice husk (100%) (e), and biochar of cow bone
(100%) (F). The mixed of rice straw (70%) + azolla
(10%) + chicken egg shell (10%) + poultry manure
(10%) was the best carrier to increase population of
T. asperellum up to 8 weeks of storage. The carbon
and nitrogen content of the carrier medium
influenced the increase cell density of T. asperellum.
REFERENCES
El-Fattah, D.A.A, Wedad E. Eweda, Mona S. Zayed,
Mosaad K. Hassanein. 2013. Effetct of Carrier
Materials, Sterilization Method and Storage
Temperature on Survival and Biological Activities of
Azotobacter chroococcum Inoculant.
Firdausi, N, Wirdhatul Muslihatin, dan Tutik Nurhidayati.
2016. Pengaruh Kombinasi Media Pembawa Pupuk
Hayati Bakteri Pelarut Fosfat Terhadap pH dan Unsur
Hara Fosfor dalam Tanah. Jurusan Biologi, Fakultas
Matematika dan Ilmu Pengetahuan Alam Institut
Teknologi Sepuluh Nopember.
Gabriel B.P & Riyatno. 1989. Metarhizium anisoplae
(Metch) Sor: Taksonomi, Patologi, Produksi, dan
Aplikasinya. Jakarta: Direktorat Perlindungan
Tanaman Departemen Pertanian.
Gusnawaty HS, Muhammad Taufik, La Ode Santiaji
Bande, dan Agus Asis. 2017. Efektivitas Beberapa
Media Untuk Perbanyakan Agens Hayati Trichoderma
sp. Jurusan Agroteknologi, Fakultas Pertanian
Universitas Halu Oleo.
Parman. 2007. Pengaruh Pemberian Pupuk Organik Cair
Terhadap Pertumbuhan dan Produksi Tanaman.
Buletin Anatomi dan Fisiologi Vol. XV, No. 2.
Prabowo AKE, Prihatiningsih N, & Soesanto L. 2006.
Potensi Trichoderma harzianum dalam
Mengendalikan Sembilan Isolat Fusarium oxysporum
Schelecht. f.sp. zingiberitrijillo pada Kencur. J. Ilmu-
Ilmu Pertanian Indonesia (2): 76–84.
Putri, SM, Iswandi Anas, Fahrizal Hazra, dan Ania Citra
resmini. 2010. Viabilitas Inokulan Dalam Bahan
Pembawa Gambut, Kompos, Arang Batok dan Zeolit
yang disterilkan dengan Iradiasi Sinar Gamma Co-60
dan Mesin Berkas Elektron. Departemen Ilmu Tanah
dan Sumberdaya Lahan Fakultas Pertanian. IPB.
Rao. 1982. Biofertilizers in Agriculture. Oxford & IB
Publishing Co. Oxford.
Ratanaphadit K, Kaewjan K, & Palakas S. 2010. Potential
of Glycoamylase and Cellulase Production Using
Mixed Culture of Aspergillus niger TISTR 3254 and
Trichoderma reesei TISTR 3081.KKU Res J. 15(9):
833–842.
Rinata, I Gede Made Adi. 2016. Pengaruh Dosis Aplikasi
Pupuk Trichokompos terhadap Pertumbuhan,
Produksi, dan Kualitas Tanah pada Tanaman Jagung
Manis (Zea mays var. saccharata Sturt) Kultivar
Talenta. Fakultas Pertanian. Universitas Lampung.
Table 3: Increased Cell Density of Trichoderma asperellum in Carrier Materials per 2 weeks Storage Period
Carrier
Increased Cell Density per 2 Weeks Storage Period (10
7
cells / g carrier)
2 – 4
4 – 6
6 – 8
A
9.55
31.40
189.38
B
17.40
90.68
209.25
C
4.50
35.84
187.13
D
0.31
12.33
62.69
E
0.13
17.46
73.13
F
0.09
22.29
116.38
ICOSTEERR 2018 - International Conference of Science, Technology, Engineering, Environmental and Ramification Researches
24

Sabrina, T., Poltakdan A. S. Hanafiah. 2017.5 Jamur Asal
Potongan Batang Sawit :Jenis dan Potensinya. USU
Medan.
Tombe, M. 2008. Sekilas Pupuk Hayati. Direktorat
Perbenihan dan Sarana Produksi.
Widyastuti SM, Sumardi, & Sumantoro P. 2001.
Efektifitas Trichoderma spp. sebagai Pengendali
Hayati Terhadap Tiga Patogen Tular Tanah pada
Beberapa Jenis Tanaman Kehutanan. J.
PerlindunganTanaman Indonesia 7(2): 98–107.
Trichoderma asperellum Cell Density in Several Carriers
25
