
Simulation of Polymer Exchange Membrane Fuel Cell Utilizing
Empty Fruit Bunch Pyrolysis using Aspen Plus
Taufiq Bin Nur
1,2
, Zulkarnaen Pane
3
, Rulianda Purnomo Wibowo
4
and Nurhayati
5
1
Department of Mechanical Engineering, Faculty of Engineering, Universitas Sumatera Utara, Padang Bulan, Medan
20155, Indonesia
2
Sustainable Energy and Biomaterial Center of Excellence, Universitas Sumatera Utara, Padang Bulan, Medan 20155,
Indonesia
3
Department of Electrical Engineering, Faculty of Engineering, Universitas Sumatera Utara, Padang Bulan, Medan 20155,
Indonesia.
4
Department of Agribusiness, Faculty of Agriculture, Universitas Sumatera Utara, Padang Bulan, Medan 20155,
Indonesia
5
Department of Industrial Engineering, Faculty of Engineering, Universitas Sumatera Utara, Padang Bulan, Medan 20155,
Indonesia.
Keywords: Polymer Exchange Membrane Fuel Cell, Empty Fruit Bunch, Pyrolysis, Aspen Plus, Biomass
Abstract: Indonesia has a large potential of biomass energy which can be used to increase electrification ratio of the
country. One the most important biomass resources to be considered as a promising option for fossil fuel
substitution and greenhouse effect reduction in the country is waste from palm oil mill plant (POM). This
study analysed the possible layout and performance of an integrated biomass pyrolysis with a polymer
electrolyte membrane fuel cell (PEMFC) as an alternative for energy system. The PEMFC is considered to be
one of a promising conversion technology for clean and efficient power generation in the current situation.
The biomass from empty fruit bunch (EFB) sent to pyrolysis unit to produce syngas which can be used as fuel
for PEMFC. A Purification processes consisting of a water gas shift reactor and a selective oxidation reactor
is necessary in order to reduce the impurity that can harmful fuel cell. It was found that, the PEMFC can
generate electricity around 512.5 kW (AC) at 0.22 A.cm
-2
with the system efficiency of 55.26% (HHV).
1 INTRODUCTION
Fuel cell is a device that converts chemical energy
into electrical energy with high efficiency through
electrochemical reaction (Guan, 2008). Fuel cell
systems have different variables such as type of the
electrolyte used in fuel cell, type of the reactants (e.g.
primary fuels and oxidants), operating temperature
and pressure, type of the exchanged ion through the
electrolyte, direct and indirect usage of the primary
fuels in fuel cell system. Based on the electrolyte
used, fuel cells can be classified into: (1) alkaline fuel
cells (AFC), (2) phosphoric acid fuel cells (PAFC),
(3) polymer electrolyte membrane fuel cell (PEMFC),
(4) molten carbonate fuel cells (MCFC), (5) solid
oxide fuel cells (SOFC) (Peighambardoust, 2010).
The polymer electrolyte membrane fuel cell
(PEMFC), with electrolyte is a solid polymer in
which protons are mobile, has received growing
attention as an efficient power generation unit due to
its low emissions, potentially high energy density,
compactness, modularity, light weight, fast start-up
and fast response to load changes (Chutichai, 2013;
Jo, 2017). The ideal fuel for PEMFC is hydrogen
which does not exist in nature and need to be
produced from other sources, such as natural gas,
water, and biomass. Due to the low temperature, the
PEMFC operates only with hydrogen of high purity,
and the concentration of carbon monoxide in the
gaseous flux should not exceed 10 ppm (Authayanun,
2013).
Biomass possesses a potential source for
renewable hydrogen production, and likely will give
the fuel cell a sustainable future. Biomass may be
divided into two groups according to its physical
characteristics: (1) liquid biomass mainly from
manure, agriculture and sludge from municipal
wastes and (2) solid biomass mainly as forest residues
Nur, T., Pane, Z., Wibowo, R. and Nurhayati, .
Simulation of Polymer Exchange Membrane Fuel Cell Utilizing Empty Fruit Bunch Pyrolysis using Aspen Plus.
DOI: 10.5220/0010079102370242
In Proceedings of the International Conference of Science, Technology, Engineering, Environmental and Ramification Researches (ICOSTEERR 2018) - Research in Industry 4.0, pages
237-242
ISBN: 978-989-758-449-7
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
237

from the forest industry (Guan, 2015). Pyrolysis is
one of the most promising technologies of biomass
utilization, and it is also the first stage of biomass
thermochemical conversion, which converts biomass
resource to solid char, liquid oil, and hydrogen-rich
gas (Yang, 2006).
Palm oil has been one of the success stories of the
North Sumatera Province of Indonesian agricultural
sector. Following the palm oil extraction procedure,
palm oil wastes are obtained as empty fruit bunch
(EFB), palm kernel shell (PKS) and palm fibre.
Approximately 1 ton of EFB, 0.3 ton of PKS, and 0.7
ton of palm fibre are produced as palm oil mill (POM)
wastes from each ton of oil production (Nizamuddin,
2016). Annual production of palm oil in Indonesia
during 2016 reached 31.40 million tons which can be
used as renewable energy sources to generate
electricity (BPS, 2017). This paper presents the
thermodynamic analysis of PEMFC fuelled by syngas
from empty fruit bunch (EFB) pyrolysis to generate
electricity by using Aspen Plus simulation.
2 SYSTEM DESCRIPTION AND
MODELLING SIMULATION
The system configuration used in this analysis
consists of pyrolysis unit, fuel reforming unit, and
PEMFC unit. The process flow diagram (PFD) of
biomass pyrolysis unit is shown in Figure 1. The
biomass combustion will be simulated at near
atmospheric condition. The Aspen Plus block units
used to simulate biomass pyrolysis process are
RYield, RGibbs, and SSplit (Nur, 2018). The biomass
(1BIOMASS) was sent to RYield, labeled with
DECOMP, to predict the decomposition of the
biomass into the reference components such as C, H
2
,
S, O
2
, N
2
, etc. The RGibbs, labelled with PYROLYS,
used as pyrolysis reactor with the nitrogen as inert
gas.
Figure 1: The process flow diagram of biomass pyrolysis
unit.
The raw-syngas (stream 5) produced by pyrolysis
unit will go to the fuel reforming unit which
comprises syngas blower (named with BLOWE3) to
increase the pressure of raw-syngas, methane steam
reforming with water gas shift reactor
(REFORMER), heat exchanger (HEX-02), water
pump (WATER-P), and a selective oxidation reactor
(SELOX). Since the raw-syngas contains high CO
fraction, which will poison the PEMFC catalysts and
then degrade the PEMFC performance. Therefore, the
system equipped with a CO removal processes as
shown in Figure 2. The CH
4
is converted to H
2
, CO
and CO
2
, and the ratio of steam to carbon (S/C) is
3.4:1. The steam obtained by utilizing the heat energy
contained in the system during the process takes place
that is by using heat exchanger during the process of
reducing the syngas temperature to the working
temperature of PEMFC. The steam methane reaction
process in the reformer reactor is followed by a CO
removal process. There are two sub-steps included in
the CO removal process, e.g., water shift reactor, and
a selective oxidation reactor (SELOX). The involved
reactions for the production of hydrogen and CO
removal are shown in table 1. remember that all the
papers must be in English and without orthographic
errors.
Figure 2: The process flow diagram of fuel reforming unit.
Table 1: Reactions involved in the hydrogen production
process and CO removal (Guan, 2015).
Steam reforming:
CH
4
+ H
2
O CO + 3H
2
∆𝐻
= 206 kJ mol
-1
CO water shift:
CO + H
2
O CO
2
+ H
2
∆𝐻
= -41 kJ mol
-1
SELOX:
2CO + O
2
2CO
2
∆𝐻
= -283 kJ mol
-1
Electrochemical:
𝐻
1/2𝑂
→𝐻
𝑂
DECOMP
PYROLYS
SLAG-REM
CALCULATO R
COMBUST
1BIOMASS
3
2
SLAG
HEATCOMB
Q-COMB
Q
4
INER T G AS
COMBUST
COMBUST
5
SYNBLO
W-SYNBLO
W
ICOSTEERR 2018 - International Conference of Science, Technology, Engineering, Environmental and Ramification Researches
238
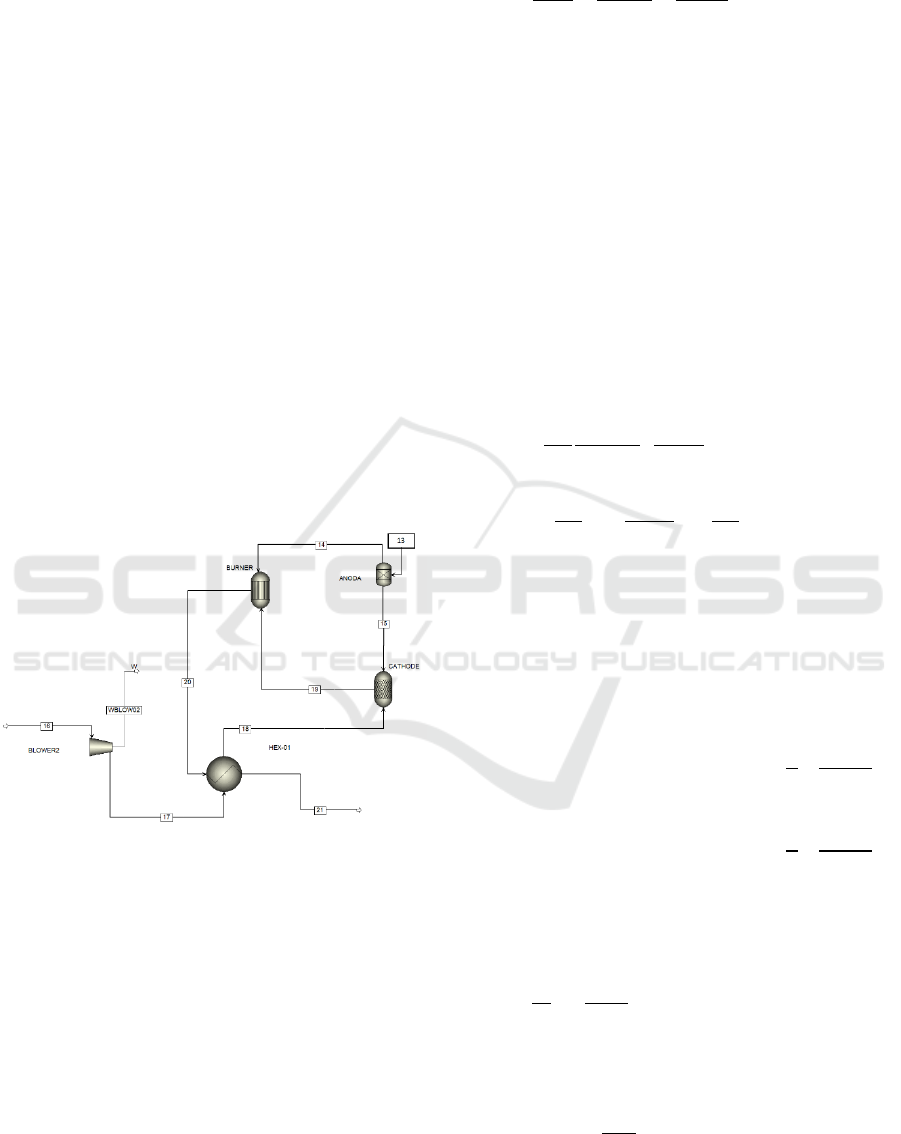
Syngas that are free from contaminants (stream
13) are fed into PEMFC, undergoing electrochemical
reactions while producing electrical energy and
thermal energy. Syngas out of the PMFC unit goes to
the burner sub unit where the remaining gases will be
burned completely. The Aspen Plus flowsheet of the
PEMFC unit is illustrated in Figure 3. The model is
based on the following assumptions: steady state
operation, pressure drops are neglected; chemical
reactions such as reforming and shift reactions reach
chemical equilibrium; the mobile ion cross over
through the electrolyte cannot modelled within Aspen
Plus, therefore the overall oxidation of H
2
was
considered instead of the cell half reaction.
3 METHODOLOGY
3.1 PEMFC Stack
The output voltage of a single cell (V
cell
) can be
obtained by considering the ohmic, activation, and
concentration losses from the thermodynamic
equilibrium potential, V
Nernst
, as follow (Jo, 2017):
Figure 3: The process flow diagram of PEMFC unit.
𝑉
𝑉
𝑉
𝑉
𝑉
(1)
where V
ohm
, V
act
, and V
con
represent the ohmic,
activation, and concentration polarization,
respectively. The Nernst Potential, V
Nernst
, is given by
(Jo, 2017):
𝑉
𝑖
𝑅
𝑅
(3)
where the area specific resistance due to the proton
transport, R_(H^+ )was obtained by considering the
membrane and catalyst layer properties below (Jo,
2017):
𝑅
.
.
.
.
(4)
The 𝑣
and 𝑣
represent the volume fractions
of the electrolyte in the anode and cathode catalyst
layers, respectively. The number 0.5 appearing in the
numerator of Eq. (4) is due to the assumption that the
average proton transport path through the catalyst
layer is half of its thickness.
The activation polarization is the voltage over
potential required to overcome the activation energy
of the electrochemical reaction on the catalytic
surface. This type of losses dominates at low current
density. The activation polarization, 𝑉
, is
calculated using the Butler-Volmer equations for
hydrogen oxidation reaction in the anode (𝑉
,
) and
oxygen reduction reaction in the cathode (𝑉
,
), as
follows (Jo, 2017):
𝑉
,
,
,
/
(5)
𝑉
,
𝑙𝑛
,
/
.
(6)
where 𝐶
and 𝛼 represent the molar concentration and
transfer coefficient, respectively. The exchange
current density of hydrogen oxidation reaction, 𝑖
,
,
and exchange current density of oxygen reduction
reaction, 𝑖
.
, can be calculated by (Jo, 2017; Jiao,
2010):
𝑖
,
𝑇
𝑖
,
353.15 𝐾
.exp1400
1
𝑇
1
353.15
(7)
𝑖
,
𝑇
𝑖
,
353.15 𝐾
.exp7900
1
𝑇
1
353.15
(8)
The concentration polarization, 𝑉
, can be
calculated by following by (Jo, 2017; Jiao, 2010):
𝑉
𝑙𝑛
(9)
where the limiting current density, 𝑖
, determined
by:
𝑖
𝑣
.
𝐷
(10)
Simulation of Polymer Exchange Membrane Fuel Cell Utilizing Empty Fruit Bunch Pyrolysis using Aspen Plus
239

After calculating the voltage losses, the fuel cell
power output is the product of the cell voltage and the
current. The total current (I) and the direct current
(DC) output power of each cell can be calculated as
follows (Taufiq, 2015; Zhang, 2005):
𝐼
,
,
.
(11)
W
𝑉
𝑥 𝐼
(12)
The alternating current (AC) power of the cell
module can be specified using (Taufiq, 2015; Zhang,
2005):
W
W
𝑥 𝜂
(13)
where 𝜂
is the inversion efficiency of direct to
alternating current.
The performance of this system is defined by its
ability to convert the chemical energy contained in
biomass into electrical. The electrical efficiency of
the system is defined as follow (Chutichai, 2013):
𝜂
𝑁𝑒𝑡 𝑝𝑜𝑤𝑒𝑟𝑒𝑑 𝑔𝑒𝑛𝑒𝑟𝑎𝑡𝑒𝑑 𝑘𝑊 𝑚
𝑥 𝐻𝐻𝑉
⁄
(14)
3.2 Input Data
EFB were selected as the main fuel in this study
because of its abundant availability in Indonesia,
especially the province of North Sumatera. The feed
composition of the EFB is specified as described in
table 2, while the main assumptions for analysis are
shown in table 3.
Table 2: Composition of EFB (Wijono, 2014).
Proximate analysis (wt.%)
Fixed carbon 9.94
Volatile matter 42.20
Moisture content 44.60
Ash content 3.26
Ultimate analysis (wt.%)
Ash content 3.26
Carbon 26.94
Hydrogen 3.22
Sulphur 0.05
Nitrogen 0.35
Oxygen 21.58
Heating values (MJ/kg)
Higher heating value (HHV) 10.29
Table 3: Main operational conditions and
assumptions for plant calculation.
EFB feed pyrolysis reactor (kg hr
-1
) 250
Nitrogen feed pyrolysis reactor (kg
hr
-1
)
25
Pyrolysis working temperature (C) 650
Pyrolysis working pressure (bar) 1.013
Environment temperature (C) 25
PEMFC operating temperature (C) 120
Anode/Cathode inlet pressure (bar) 1.20
Thickness of anode/cathode GDLs,
CLs, 𝛿
, 𝛿
(mm) (Jo, 2017)
0.35;
0.015
Thickness of anode/cathode
membrane, 𝛿
(mm) (Jo, 2017)
0.07
Reference H
2
/O
2
molar
concentration, 𝐶
,
/𝐶
,
(mol/m
3
) (Jo, 2017)
40.88
Anode/cathode transfer coefficient
(Jo, 2017)
0.5; 0.65
Reference exchange current density
in anode/cathode, 𝑖
,
, 𝑖
,
(A/m
2
)
(Jo, 2017)
1.0 x 10
9
;
1.0 x 10
4
Porosity of GDL, CL (Jo, 2017) 0.6; 0.4
Volume fraction of ionomers in CL
(Jo, 2017)
0.3
Electronic conductivity in CL (S m
-
1
) (Jo, 2017)
300
DC to AC inverter efficiency (%) 95
Electric generator efficiency (%) 98.7
Miscellaneous BOP, % input HHV 13.3
4 RESULTS AND DISCUSSION
The main raw synthesis gas mass and composition
leaving the pyrolysis unit (stream 4) are 264.20
kg.hr
-1
and 13.74% H
2
O, 42.92% H
2
, 6.68% N
2
,
0.04% S, 19.61% CO, 12.88% CO
2
, 4.13% CH
4
,
respectively. This raw synthesis gas from the
pyrolysis process is treated to reduce the quantity of
CO it contains to an acceptable level for PEMFC
operation. The main raw syngas composition leaving
the reformer and CO removing are 21.8% H
2
O, 1.4
O
2
, 31.8% H
2
, 24.8% N
2
, 0.0143% S, 19.7% CO
2
,
0.4394% CH
4
. Then, this clean syngas goes to the
PEMFC unit.
The performance of the PEMFC is evaluated
using a polarization curve showing the relationship
between current density, cell potential and power
density. The cell potential decreases with increasing
current density due to large voltage losses are
observed at higher current density as shown in figure
ICOSTEERR 2018 - International Conference of Science, Technology, Engineering, Environmental and Ramification Researches
240

4. It can be seen that the activation polarization is
much higher than the other polarizations.
The maximum power density that PEMFC unit
can generate in this study is around 0.829 W.cm
-2
at
current density of 1.42 A.cm
-2
as shown in figure 5.
With the total current is 502,932.72 A, the PEMFC
unit can produce power of 512.5 kW (AC) at 0.22
A.cm
-2
.
Based on the analysis, it was observed also the
requirement of power during processes within the
system and system electrical efficiency. Those
internal power consumed are listed in table 4. The
system electrical efficiency when PEMFC generate
power of 512.5 kW (AC) at 0.22 A.cm
-2
is 55.26%
(HHV).
Figure 4: PEMFC voltage characteristics versus current
density.
Figure 5: Effect of current density of voltage and power
density.
Table 4. Energy consumption during processes
Energy consumed by blower to supply
air for SELOX reactor (BLOWER3)
1.72 kW
Energy consumed by syngas blower
(
SYNBLO
)
7.04 kW
Energy consumed by air blower for
cathode section (BLOWER2)
12.33 kW
Energy consumed by water pump
(WATER-P)
0.0035 kW
Miscellaneous BOP 96.47 kW
5 CONCLUSIONS
In this study, the system consists of a biomass
pyrolysis to produce syngas as fuel and PEMFC to
generate electricity was analysed by using Aspen Plus
simulation. It was found that EFB is a potential fuel
for PEMFC unit. When this biomass pyrolysis unit
operated at 650 C and atmospheric pressure, it can
produce raw syngas which composition of H
2
is
42.92%. Based on the analysis, the PEMFC can
generate electricity around 512.5 kW (AC) at 0.22
A.cm
-2
while the system efficiency can reach up to
55.26% (HHV). More details calculation doing
modelling simulation and experimental are needed to
improve this analysis.
ACKNOWLEDGEMENTS
This work has been fully supported by Directorate of
Research and Community Service, Directorate
General Strengthening Research and Development
Ministry of Research, Technology and Higher
Education Republic of Indonesia, in accordance with
the funding agreement and community service for
fiscal year 2018.
REFERENCES
Authayanun S, Mamlouk M, Scott K, Arpornwichanop A
2013 Applied Energy 109 pp 192-201
BPS Statistics Indonesia 2017 Indonesian Oil Palm
Statistics 2016
Chutichai B, Authayanun S, Assabumrungrat S,
Arpornwichanop A 2013 Energy 55 pp 98-106
Guan T, Alvfors P 2015 Energy Procedia 75 pp 2003-2008
Guan T, Chutichai B, Alvfors P, Arpornwichanop A 2015
Energy Conversion and Management 106 pp 1183-
1191
Jiao K, Li X 2010 Fuel Cells 10 pp 351-362
0,00
0,20
0,40
0,60
0,80
1,00
1,20
0,00 0,20 0,40 0,60 0,80 1,00 1,20 1,40 1,60 1,80
CellVoltage(V)
CurrentDensity(A/cm
2
)
OhmicPolarization
ActivationPolarization
ConcentrationPolarization
CellVoltage
0,00
0,10
0,20
0,30
0,40
0,50
0,60
0,70
0,80
0,90
0,00
0,10
0,20
0,30
0,40
0,50
0,60
0,70
0,80
0,90
1,00
1,10
1,20
1,30
0,0 0,2 0,4 0,6 0,8 1,0 1,2 1,4 1,6 1,8
PowerDensity(Watt/cm
2
)
CellVoltage(Volt)
CurrentDensity(A/cm
2
)
Simulation of Polymer Exchange Membrane Fuel Cell Utilizing Empty Fruit Bunch Pyrolysis using Aspen Plus
241

Jo A, Oh K, Lee J, Han D, Kim D, Kim J, Kim B, Kim J,
Park D, Kim M, Sohn Y J, Kim D, Kim H, Ju 2017
International Journal of Hydrogen Energy 42 pp 1698-
1714
Nizamuddin S, Shrestha S, Athar S, Ali B S, Siddiqui M A
2016 Rev Chem Eng 32 pp 489-505
Nur T B, Syahputra A W 2018 IOP Conf. Series: Materials
Science and Engineering 308
Peighambardoust S J, Rowshanzamir S, Amjadi M 2010
International Journal of Hydrogen Energy 35 pp 9349-
9384
Taufiq B N, Kikuchi Y, Ishimoto T, Honda K, Koyama
2015 Applied Energy 147 pp 486-499
Wijono A 2014 Proceeding Simposium Nasional RAPI
XIII-2014 FT UMS (Indonesian Version).
Yang H, Yan R, Chen H, Lee D H, Liang D T, Zheng C
2006 Energy & Fuels 20 pp 1321-1328
Zhang W, Croiset E, Douglas P L, Fowler M W, Entchev E
2005 Energy Conv Manag 46 pp181–96
ICOSTEERR 2018 - International Conference of Science, Technology, Engineering, Environmental and Ramification Researches
242
