
The Potency of Plant Growth Promoting Rhizobacteria (PGP
R
) of
Coastal Poaceae (Phragmites karka) to Stimulating of Paddy (Oryza
sativa L.) Growth
Yurnaliza
1
, Artha Joseva Hutapea
1
and Nunuk Priyani
1
1
Departement of Biology, Faculty Mathematics and Natural Sciences, Jl. Bioteknologi No. 1, Kampus USU, Universitas
Sumatera Utara, Medan, 2015, Indonesia.
Keywords: Coastal area, Paddy, PGPR, Phragmites karka.
Abstract: The aim of this research was to obtain and to observe the activity of plant growth promoting rhizobacteria
(PGPR) isolated from coastal Poaceae, Phragmites karka, on stimulating paddy’s growth. The bacteria were
qualitatively on three different selective media: Pikovskaya, JNFb, and tryptophan containing LB media to
observe the ability of bacteria to solubilize phosphate, to fix nitrogen, and to produce indol acetic acid (IAA).
The selected potential bacteria from each test were studied further as PGPR candidates on stimulating paddy
growth in sterilized soil medium. The results showed that there were 6 isolates able to solubilizing phosphate,
6 isolates capable to fixing nitrogen and 8 isolates able to producing IAA. The synergy test resulted in three
selected isolates (PP03, RG08 and PI05) were synergistic each other. The application of three isolates as
single culture and their consortium stimulated of paddy growth significantly comparing to control (without
bacteria) especially in the plant height and root length parameter. The best performance of paddy growth was
achieved from consortium of 3 bacterial isolates treatment.
1 INTRODUCTION
Indonesia is one of the most populous countries in
the world. Rapid population growth leads to increase
in food demand. Society and government use various
measures to maintain food availability. Food
production is needed to improve of food demand
fulfillment. Efforts that have been made to increase
food production still need to be improved. One effort
that can be done is extended farmlands by utilized
marginal land such as coastal area. Marginal land is
a nutrient-poor land with environmental conditions
do not support the plant growth. Coastal area have
sandy soils and high salinity content. Only tolerance
organism can grow in this area. Indonesian Centre for
Rice Research have breeding program to improve and
find a salinity tolerance of rice (Hairmansis et al., 2017).
Beside the plant tolerant to salinity, other efforts to
make marginal land become usable are attempted the
organism plant growth promoting tolerance to
salinity.
Plant Growth Promoting Rhizobacteria (PGPR)
was a numerous soil bacteria which occupy roots or
rhizosphere area, stimulating plant growth in various
mechanism and also induced plant resistance
(
Vessey,2003). Investigation of PGPR microorganism
from many sources is the purpose to making it as
biofertilizers. In association of plant and PGPR,
microorganism given some benefit action to
stimulating plant growth specifically by fixing
dinitrogen from atmosphere, solubilizing unsoluble
phosphate, producing growth hormone of Indole-3-
acetic acid (IAA), antimicrobial compound to
reduced plant pathogen and induced plant resistance
(Vessey, 2003), (Sandilya et al., 2016). Bacteria
Burkholderia cepaciai is one of species PGPR
bacteria that potential as stimulated growth of maize
and also have biocontrol activity to Fusarium spp.
(Bevivino et al., 1998).
Many PGPRs are diazotrophs (symbiosis or non
symbiosis nitrogen fixing bacteria). Many
diazotrophs bacteria are phosphate solubilizing and
IAA producer (Vessey, 2003). In lower conditions
of N or P level in soil, diazotroph microorganism
adapt in the rhizosphere but harmless to their host.
Population and activity of microbial in rhizosphere of
sandy soil land is more higher than in rhizosphere of
humus soil, and it’s call as rhizosphere effect
Yurnaliza, ., Hutapea, A. and Priyani, N.
The Potency of Plant Growth Promoting Rhizobacteria (PGPR) of Coastal Poaceae (Phragmites karka) to Stimulating of Paddy (Oryza sativa L.) Growth.
DOI: 10.5220/0010088600670072
In Proceedings of the International Conference of Science, Technology, Engineering, Environmental and Ramification Researches (ICOSTEERR 2018) - Research in Industry 4.0, pages
67-72
ISBN: 978-989-758-449-7
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
67

(Dotaniya and Meena, 2015). Low land moisture such as
sandy soil affect microorganism to move toward the
plant root zone. Availability nutrient in rhizosfer
influence the rhizosphere population microorganism
(
Dotaniya and Meena, 2015).
In this research will be found a new candidate the
plant growth promoting rhizobacteria of Phragmites
karka which collected from coastal area.
2 MATERIAL AND METHODS
2.1 Material Research
The soil from Phragmites karka rhizosphere were
collected as much as 100 g from Coastal location in
Percut Sei Tuan area. The soil was collected from 3
samples point and stored on sterile of sealed plastics
sample before transfer to laboratory. Seed of paddy
was obtained from commercial seed seller. Several
medium Pikovkayas, JNFb (medium free nitrogen
source) and Luria Bertani (LB)+ Tryptophan medium
were used for cultivation and selection of PGPR
activity.
2.2 Isolation of PGPR Bacteria
The amount of 1 g of soil sample was diluted into 9
ml of sterile distilled water and than homogenized.
Serial dilution was conducted to obtain a 1x 10
-5
dilution. A total of 0.1 ml of soil suspension from the
last dilution was spread to the surface of Pikovskaya,
JNFb and LB + tryptophan medium aseptically. The
culture medium was incubated for 2-3 days at room
temperature. Activity of phosphate solubilizing
bacteria were detected qualitatively by clear zone
formation around colony of bacterial on pikovskaya
medium. The positive test of nitrogen-fixing bacteria
were characterized by the presence of colonies
bacteria on JNFb medium. The medium of JNFb was
free nitrogen source. Presence of bacteria in JNFb
medium indicated the bacteria can fix bacteria from
atmosphere. Bacteria produced IAA in medium LB +
tryptophan broth medium were checked by added
Salkowski’s reagent (2% 0.5M FeCl
3
in 35% HClO
4
solution). The color medium become purple if there
was indol compound in the medium. The positive sign
bacteria growth in selective medium were purified
and further characterized.
2.3 Selection of Phosphate Solubilizing
Bacteria
The purified bacteria isolates were further tested
for their ability to dissolve the phosphate to
determine its solubility index, fixing nitrogen and
produce IAA. Phosphate solubility index (PSI) score
was obtained from the comparison of the diameter of
clear zone around the bacterial colony and diameter
of colony bacteria (Dotaniya and Meena, 2015).
2.4 Selection of Nitrogen Fixing
Bacteria
Nitrogen fixation from purified bacteria were
obtained from pellicle size formed of bacteria when
cultured on semi solid JNFb medium. The white
pellicle formed will be visible in the surface medium
on tube and measured after 10 days incubation at
room temperature.
2.5 Selection of IAA Producing
Bacteria
The IAA produced by each bacteria was measured
with colorimetric technique using the Salkowski’s
method (Ehmann, 1977). Bacterial suspension (3 ml)
with cell density in Optical Density (OD) 600 was
0.5 (≈ 10
8
Colony Forming Unit, CFU / ml) was
introduced into 27 ml of liquid LB + tryptophan broth
and incubated at 28°C for 6 days and shaken at 100
rpm. Every 2 days as much as 10 ml of culture fluid
is taken and then centrifuged at 5500 rpm for 10
minutes. The supernatant was transferred to a new
sterile tube and then added Salkowski reagent with 4
: 1 ratio (supernatant : salkowski) and incubated for
20 minutes at room temperature. Colorimetrically the
color change formed was measured by a
spectrophotometer at a wavelength of 535 nm. The
concentration of IAA from the sample was calibrated
from linear regression equation of pure IAA.
2.6 Application of Selected Bacteria to
Improved Paddy Growth
Paddy seeds were cultivated on 1 kg of sterile humus-
sandy soil with composition humus: sandy soil (3:1)
for 1 week. The potential bacteria were checked their
synergistic each other by cross inoculated onto agar
medium. When two bacteria was in synergism
condition, they can live together with no inhibited
each other. The selected bacteria was cultivated on
Nutrient Broth for 24 hours. Ten milliliter of bacterial
ICOSTEERR 2018 - International Conference of Science, Technology, Engineering, Environmental and Ramification Researches
68

culture broth OD 600 (0,5) or in consortium form
were poured into rhizosphere of paddy seedling on
soil medium. Each treatment was 3 replications.
Parameters of paddy growth were analyzed as plant
height, root length, biomass of plant and how much
the dead plant. Observation of rice plant growth was
done at 30 days after planting.
3 RESULTS AND DISCUSSION
3.1 Isolation Results
A total of 20 bacteria were isolated from Phragmites
karka rhizosphere. Six isolates were each growth on
Pikovkaya and JNFB medium and also 8 isolates on
LB+Tryptophan medium. bacteria and 8 isolates are
IAA producing bacteria. Based on the colony
morphology observations showed that the 20 isolates
had various characteristics in shape, edge, elevation
and color. The colony shapes were circular, irregular
and rhizoid. Almost all isolates were had white color
and several cream. Only one isolate had orange color
(Table 1).
3.2 Phosphate Solubilizing, Nitrogen
Fixing and IAA Producing Bacteria
From 20 isolates bacterial tested were only 7
isolates potential to give positive results to all tree
test. Results for phosphate solubilizing activity
showed that 14 isolates bacterial were able to dissolve
phosphate and one isolate with the highest ability was
PP03. In the nitrogen fixing test was indicated by
pellicle size showed that only 9 isolates produced the
pellicle when growth on semisolid medium of JNFb
and seven isolates produced thick pellicle with the
size 5-9 mm. All isolates tested were IAA producer,
and only two isolates produced IAA in concentration
rage 28-40 ppm i.e. PP03 and RG08. Seven bacterial
isolates were only dissolved phosphate and produced
IAA and two isolates were only fixed nitrogen and
produced IAA. From all tested bacteria, four isolates
had one ability that only as IAA producer (Table 2).
Three isolates bacteria which have best perform to all
selected criteria were collected i.e. PP03, RG05 and
PI05) and then used in paddy growth application. The
synergistic test results showed that tree bacteria
synergistically (Table 1).
Three selected bacteria in this research were potential
as biofertilizer candidate. Existence of
microorganisms as biofertilizer increases the growth
of plants by enrichment of soil nutrients (nitrogen
fixation) or making nutrient like phosphate more
available to plant or increasing number of roots to be
increasing nutrient absorption from soil or producing
growth hormone to stimulated plant growth (
Vessey,
2003). PGPR may
Table 1: Morphological character of colony bacteria from Phragmites karka, cultivated on 3 different agar medium
No Code Agar medium Shape Edge Elevation Color
1 PP01 Pikovskaya Agar Irregular Entire Flat White
2 PP02 Pikovskaya Agar Irregular Undulate Flat White
3 PP03 Pikovskaya Agar Circular Filamentous Raised White
4 PP04 Pikovskaya Agar Circular Undulate Raised White
5 PP05 Pikovskaya Agar Circular Entire Flat White
6 PP06 Pikovskaya Agar Rhizoid Filamentous Flat White
7 RG01 JNFB Circular Entire Flat White
8 RG02 JNFB Circular Undulate Flat White
9 RG05 JNFB Rhizoid Filamentous Flat White
10 RG06 JNFB Circular Undulate Raised Orange
11 RG07 JNFB Circular Undulate Raised White
12 RG08 JNFB Rhizoid Filamentous Flat White
13 PI01 LB Irregular Filamentous Flat White
14 PI02 LB Irregular Entire Raised Cream
15 PI03 LB Circular Entire Flat White
16 PI04 LB Circular Entire Raised Cream
17 PI05 LB Irregular Filamentous Raised Cream
18 PI06 LB Irregular Entire Flat White
19 PI07 LB Irregular Filamentous Flat White
20 PI08 LB Rhizoid Filamentous Flat White
The Potency of Plant Growth Promoting Rhizobacteria (PGPR) of Coastal Poaceae (Phragmites karka) to Stimulating of Paddy (Oryza
sativa L.) Growth
69

colonize plant on rhizosphere or endophytes. Not any
soil bacteria can colonize in that area. Association
between rhizobacteria and plant were symbiotically
give benefit to plant. Non-symbiotic diazotroph
bacteria like Azospirillum, Azotobacter,
Gluconacetobacter diazotrophicus, Beijerinckia sp.
can live in rhizospher and promoting plant growth
(
Vessey,2003).
3.3 Analyzed of Paddy Growth
Application of 3 isolates bacteria to paddy seed were
observed to several plant growth parameter .
Inoculated of 3 bacteria and their consortium
treatment statistically were not give significant results
for plant biomass measured but had significantly
results than control (P0/no bacterial treatment) to
stimulated plant height and root length.
Table 2: Combination ability of phosphate solubilizing bacteria,nitrogen fixing bacteria and IAA producing bacteria
NO
Bacterial
Isolate
Phosphate
solubilizing index
Pellicle size
Consentration of
IAA
1 PP01 + - +
2 PP02 + - ++
3 PP03 +++ +++ +++
4 PP04 + - ++
5 PP05 ++ - +
6 PP06 + - +
7 RG01 + +++ +
8 RG02 + +++ +
9 RG05 + +++ +
10 RG06 + - ++
11 RG07 + +++ +
12 RG08 + - +++
13 PI01 - + +
14 PI02 - - +
15 PI03 - - +
16 PI04 - - +
17 PI05 ++ +++ ++
18 PI06 + ++ +
19 PI07 - - ++
20 PI08 - +++ +
Noted. Phosphate solubilizing index: - (0), + (1-2), + + (2-3), + + + (3-4); Pellicle Size (mm): - (0), + (1-3), + + (3-5), + +
+ (5-9); IAA Consentration (ppm): - : 0, + (1-14), ++ (15-27), + + + (28-40)
Table 3: Average paddy seedling growth parameter after 30 days planting
Treatment
Average seedling Growth Parameters
Plant
height
(cm)
Root
length (cm)
Wet
weight
canopy
(g)
Weight of
wet root
(g)
Dry weight
of canopies
Dry
weight of
root(g)
P0 (without bacteria) 35,04
a
4,71
a
0,75
a
0,20
a
0,13
a
0,07
a
P1 (PP03) 39,99
b
8,67
b
c
0,71
a
0,36
a
0,11
a
0,09
a
P2 (RG08) 39,10
b
10,50
c
0,61
a
0,35
a
0,08
a
0,09
a
P3 (PI05) 42,09
b
c
5,08
a
0,73
a
0,42
a
0,10
a
0,10
a
P4 (PP03+RG08) 41,90
b
c
9,43
b
c
0,74
a
0,37
a
0,11
a
0,10
a
P5 (PP03+PI05) 41,31
b
c
8,10
b
c
0,48
a
0,19
a
0,08
a
0,06
a
P6 (RG08+PI05) 42,55
b
c
8,44
b
c
0,67
a
0,43
a
0,09
a
0,09
a
P7 (PP03+RG08+PI05) 44,84
c
7,81
b
0,80
a
0,47
a
0,13
a
0,14
a
Description: The average value that has the same letter in the same column shows no significant difference according to the DMRT test
at the real level of 5%
ICOSTEERR 2018 - International Conference of Science, Technology, Engineering, Environmental and Ramification Researches
70
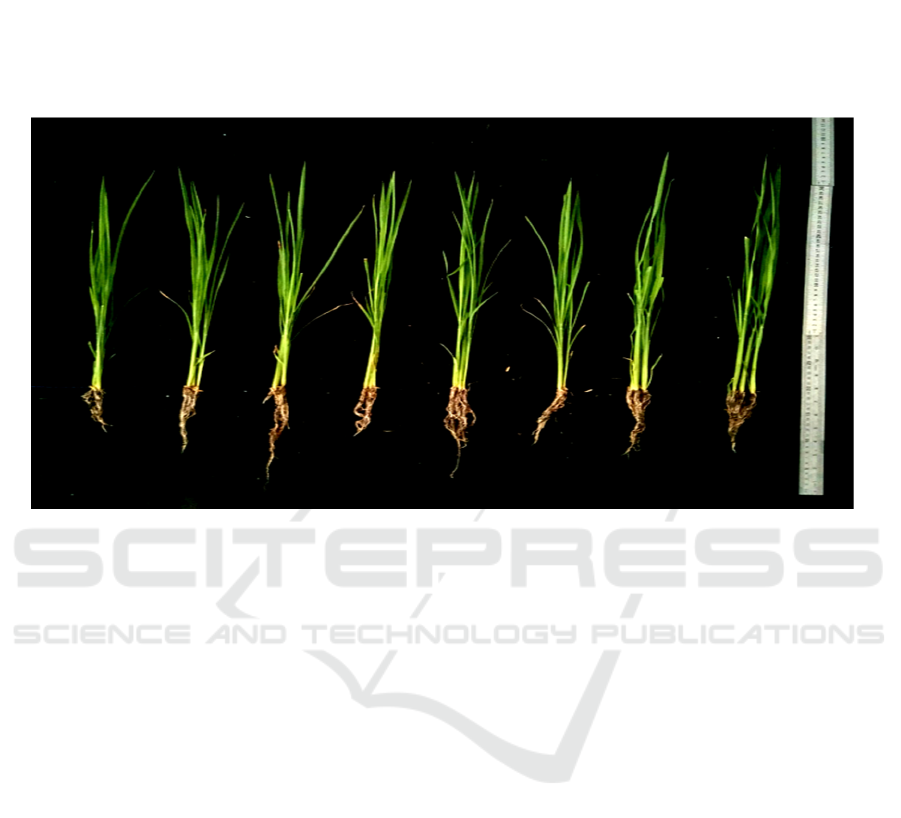
The best results for plant height was from P7
treatment and for length of roots was P2 treatment
(isolate of RG08) (Table 3). All treatment of PGPR
bacterial and their consortium provided best
performance to paddy growth than control, even
though from growth parameter P7 treatment was the
best results (Figure 1). The research about application
of biofertilizer to Sugarcane soil showed that
biofertilizer will substitute 25%-50% of NPK
fertilizer in soil (
Mulyani
et al., 2017). Inoculation of
PGPR bacteria to maize plant increased plant
performance in chlorophyll content, root and shoot
length (
Ullah et al., 2017)
.
Figure 1: Performance of paddy seedlings after treated with rhizobacteria of Phragmites karka and observed on 30 days
planting. Noted: P0 (without bacteria), P1-P7 were treated by P1 (PP03 isolate), P2 (RG08 isolate), P3 (PI05 isolate), P4
(PP03 and RG08 isolates), P5 (PP03 and PI05 isolates), P6 (RG08 and PI05 isolates) and P7 (PP03, RG08 and PI07 isolates).
4 CONCLUSION
Selective isolation of rhizobacteria from Phragmites
karka were obtain 20 isolates and were selected
three isolates as PGPR candidate. Only seven isolates
were able to fixing nitrogen, solubilizing phosphate
and producing IAA. Three selected isolates had the
best activity for all test. Application of three selected
isolates and their consortium to paddy growth
resulted best performance to paddy growth especially
for plant height and length of root than control
(without bacterial treatment).
ACKNOWLEDGEMENTS
We would to thank to head of Microbiological
laboratory, Department of Biology, Universitas
Sumatera Utara for supporting this research.
REFERENCES
Bevivino A, Sarrocco S, Dalmastri C, Tabacchioni S,
Cantale C and Chiarini L., 1998. Characterization of A
Free-Living Maize-Rhizosphere Population of
Burkholderia Cepacia: Effect of Seed Treatment on
Disease Suppression And Growth Promotion of Maize.
FEMS Microbiol. Ecol. 27: 225–237.
Dotaniya ML, Meena VD., 2015. Rhizosphere Effect on
Nutrient Availability in Soil and Its Uptake by Plants:
A Review. Proc. Natl. Acad. Sci., India, Sect. B Biol.
Sci. 85(1):1–12.
Hairmansis A, Nafisah, and Jamil A., 2017. Towards
Developing Salinity Tolerant Rice Adaptable For
Coastal Regions in Indonesia. KnE Life Sciences. 72–
79.
Mulyani O, Trinurani E, Sudirja R, and Joy B., 2017. The
Effect of Bio-fertilizer on Soil Chemical Properties of
Sugarcane in Purwadadi Subang. KnE Life Sciences.
164–171.
Sandilya SP, Bhuyan PM, Gogoi DK, Kardong D., 2016.
Phosphate Solubilization and Plant Growth Promotion
Ability of Rhizobacteria of Ricinus Communis Growing
in Assam, India. Proc. Natl. Acad. Sci., India, Sect. B
Biol. Sci springer.
P
0
P1
P2
P
3
P4
P
5
P
6
P
7
The Potency of Plant Growth Promoting Rhizobacteria (PGPR) of Coastal Poaceae (Phragmites karka) to Stimulating of Paddy (Oryza
sativa L.) Growth
71

Ullah S, Mumtaz A and Bano A., 2013. Effect of PGPR on
Growth and Performance of Zea mays. Research
Journal of Agriculture and Environmental
Management. 2(12): 434-447.
Vessey JK., 2003. Plant Growth Promoting Rhizobacteria
as Biofertilizers. Plant and Soil. 255: 571–586.
ICOSTEERR 2018 - International Conference of Science, Technology, Engineering, Environmental and Ramification Researches
72
