
Synthesis and Characterization of Carboxymethyl Cellulose using
Solvents Variations
Sri Yuliasmi
1
, Henny Sri Wahyuni
1
, Hanifah Siti Aisyah
1
and Devi Riati
1
1
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Universitas Sumatera Utara, Medan, Indonesia
Sriyuliasmi@usu. ac.id , henny@usu. ac.id
Keywords: Carboxymethyl cellulose, degree of substitution, organic solvent.
Abstract: Carboxymethyl cellulose (CMC) is a cellulose derivative which is widely used in pharmaceutical and non-
pharmaceutical industries. One of the important parameters in CMC synthesis is the variation of solvent
medium. Solvents can affect the quality of CMC. The present study was conducted to synthesize and
characterize of CMC with the best organic solvent mixture. CMC was obtained from microcrystalline
cellulose by three stages, including alkalization between cellulose and sodium hydroxide with solvent;
carboxymethylation using sodium monochloroacetate; neutralization and purification using glacial acetic
acid-methanol. The solvent medium used were isopropanol-n butanol; benzene-ethanol-air; isopropanol-
benzene; isopropanol-ethanol-air, and isopropanol-isobutanol by each variety of comparisons. CMC was
characterized by Infrared Spectrophotometry. Degree of substitution and organoleptic test were then
determined. The optimum condition of CMC synthesis which provided highest degree of substitution of 0.9
was found on isopropanol-ethanol solvents in ratio of 50:50. Organoleptic test showed that CMC powdered
was colourless, rough, odorless and tasteless. Infrared analysis revealed the presence of carboxyl and ether
functional groups in the 1600-1000 cm
-1
region. It can be concluded that CMC has been succesfully
synthesized using isopropanol-ethanol as the best solvent.
1 INTRODUCTION
One of cellulose derivatives that the most widely
used is CMC. Many industries such as food,
pharmaceutical, detergent, textile, cosmetic, and
ceramic industries have used CMC as excipient
(Koh, 2013). The study of Technavio London shows
that the needs of CMC in the world will increase
rapidly by 5% in 2017-2021 (Maida, 2017).
The increasing variety of CMC usage encourages
the production of good quality CMC synthesis.
CMC synthesis involves the conversion of cellulose
into alkaline cellulose which then hydroxyl groups
of cellulose are substituted by carboxymethyl groups
by reacting them to sodium monchloracetate (Na-
MCA) (Heinze and Pfeiiffer, 1999).
The process of CMC synthesis consists of
several steps, namely alkalization,
carboximethylation, purification, and neutralization.
Alkalization and carboxymethylation reactions are
steps that determine the value of the DS. The
alkalization process aims to stretch the
intramolecular and intermolecular hydrogen bonds
of cellulose so easily substituted into carboxymethyl
groups using NaOH in a suitable solvent. The
number of substituted hydroxyl groups is called
degree of substitution (DS) (Cash and Caputo,
2010). The solvent is used should be inert. It
facilitates NaOH to penetrate well in reaction
cellulose. The addition of NaOH is important in
producing alkaline cellulose. The solvent also
provides in sodium mono chloro acetic acid
accessibility to AGU (anhydroglucose unit) cellulose
reaction that occurs during CMC synthesis
(Stigsson, 2006).
The quality of CMC can be obtained from
several parameters. DS is the main parameter in
determining quality of CMC, the DS value also
states the solubility of CMC in water. The maximum
degree of substitution for CMC is average value
between 0.4 to 1.5. The higher degree of substitution
of CMC, the easier solubility in water
(Aambjornsson, 2015).
DS values are influenced by several factors, one
of which is the type and composition of the solvent
medium in alkalization step. The purpose of this
research is conducted to synthesize and characterize
of CMC with the best organic solvent mixture
.
Yuliasmi, S., Wahyuni, H., Aisyah, H. and Riati, D.
Synthesis and Characterization of Carboxymethyl Cellulose using Solvents Variations.
DOI: 10.5220/0010093410151020
In Proceedings of the International Conference of Science, Technology, Engineering, Environmental and Ramification Researches (ICOSTEERR 2018) - Research in Industry 4.0, pages
1015-1020
ISBN: 978-989-758-449-7
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
1015

2 METHODS
This research was conducted in two stages. First
stage includes synthesis carboxymethyl cellulose
using some solvents in different ratio. The second
stage includes characterization of the syntesized
carboxymethyl cellulose by Infra Red
Spectrophotometry, degree of substitution, and
organoleptic test.
2.1 Material
The materials used in the research were
microcrystalline cellulose, distilled water, sodium
hydroxide, sodium monochloroacetate (NaMCA),
isobutyl alcohol, N-butanol, isopropyl alcohol,
ethanol, methanol, benzene, glacial acetic acid.
2.2 Synthesis of Carboxymethyl
Cellulose
This procedures followed the method of Safitri
(2017) and Mulyatno (2017). 5 grams of cellulose
was added to 100 ml of isopropyl alcohol, and 20 ml
of NaOH while it was stirred in a temperature of
25°C for 1 hour. Then, added sodium chloroacetate
to the mixture. The mixture was then heated while
stirring at a temperature of 55
o
C for 3 hours.
Afterwards the mixture was filtered and the residue
soaked using 100 ml of methanol for 24 hours. The
mixture was neutralized using glacial acetic acid
solution and then filtered. The residue was dried in
an oven with a temperature of 60° C to a constant
weight. The formula of CMC synthesis can be seen
on Table 1.
Table 1: Design of formula synthesis of CMC.
Formula Solvents NaOH NaMCA
Isopropanol: N-
Butanol
10% 4 grams
F1 80:20
F2 50.50
F3 30:70
Benzene:Ethanol:Wat
er
F4 70:20:10
F5 50:30:20
F6 50:50:0
Benzene:Isopropanol
F7 30:70
F8 50:50
F9 70:30
Isopropanol:Isobutano
l
F10 70:30
F11 50:50
F12 30:70
Isopropanol:Ethanol:
Water
F13 70:20:10
F14 50:50:0
2.3 Characterization of Carboxymehyl
Cellulose
2.3.1 The Organoleptic Properties Test
It included colour, taste, texture and odor.
2.3.2 Degree of Substitution Determination
(DS)
Relative values of degree of substitution of carboxyl
group in CMC could be analysed by IR spectra. By
comparing absorbance of carboxyl group stretching
vibration and methine stretching vibration (Rrel
=A
1605
/A
2920
), we could evaluate the relative amount
of carboxyl group in the sample. DSrel was
calculated by the following equation (Singh and
Khatri, 2012):
DSrel = Rrel
(
CMC
)
– Rrel
(
cellulose
)
(1)
2.3.3 Infrared Spectrophotometry Analysis
All measurements were carried out using the KBr
method. The samples were dried in an oven at 60◦C.
Sample and KBr were mixed (1:100) then ground
until homogenous. Afterwards the mixture was
compressed to a form a transparent disk. The
infrared spectra of these samples were recorded with
a FT-IR Shimadzu Spectrophotometer between 400
and 4000 cm
−1
.
3 RESULT AND DICUSSION
Synthesis of CMC was began by suspending the
cellulose in a solvent using a mechanical stirrer at
room temperature. The effect of solvent which is
related to the ability of the reaction to dissolve the
etherifying agents (NaMCA) and swell the cellulose
to improve the accessibility NaMCA into cellulose
structure. The right solvent ratio will increase the
substitution of reaction, and if the ratio is not
appropriate the reaction will be inhibited (Ismail,
2010).
The next process was carboxymethylation
namely the addition of sodium monochloroacetic
ICOSTEERR 2018 - International Conference of Science, Technology, Engineering, Environmental and Ramification Researches
1016
(ClCH
2
COONa) with stirring for 3 hours at a
temperature of 55ºC. Resulting by products such as
sodium glycolate and sodium chloride at this stage.
Separating CMC from the side product with added
methanol and mixed. Neutralization of product was
conducted because the reaction process running on
alkaline conditions used acetic acid. CMC had been
cleaned and then dried using an oven at a
temperature of 60ºC (Musfiroh, 2013).
The influence of the type of solvent can be
explained in terms of polarity and stereochemistry.
Based on these properties, it is known that the
smaller the value of the polarity of the
solvent/reaction medium would increase the
effectiveness carboxymethylation (etherification)
and a smaller polarity solvent would also help keep
the cellulose molecules were less decomposed by
alkali. The formation of this layer makes the amount
of NaOH were distributed in the cellulose phase
enough to turn cellulose into cellulose-defined
shapes (Stigsson, 2006) (Pitaloka, 2017).
In other words, the polarity of the smaller
reaction medium would help the formation of alkali
cellulose with a uniform distribution. This uniform
distribution can be achieved because of low
solubility in the alkaline solution non-polar system
so that the hydroxyl group (OH) is not reactive with
the Na+. Decreased reactivity of hydroxyl groups
followed by a longer carbon chain (C) in alcohol.
Larger methyl group can make the low reactivity of
hydroxyl groups (Zhang, 1993). Polarity index of
some organic solvent is showed on Table 2.
Table 2: Data polarity index from some solvent.
Solvent Polarity Index
benzene 2.7
isopropanol 3.9
N-butanol 4
isobutanol 4
ethanol 5.2
water 9
3.1 The Organoleptic Properties
The results of the organoleptic synthesized CMC is
show in Table 3. Table 3 shows that synthesized
CMC has organoleptic with color is ivory, rough
powder, odorless, and tasteless. Overall the
synthesized CMC had similar organoleptic
caracteritics except F6 until F9. From the table it can
be seen that CMC produced using a solvent mixture
of benzene has organoleptic with lump hard and
colors are yellow to brown whereas the synthesized
CMC using a solvent mixture of water has a white
color and the form of a fine powder.
Table 3: The organoleptic properties of synthesized CMC.
Formula
Organoleptic
Colour Texture Odor Taste
F1 Ivory
Rough
powder
No No
F2 Wheat
Rough
powder
No No
F3 Ivory
Rough
powder
No No
F4 White
Fine
powder
No No
F5 White
Fine
powder
No No
F6 Ivory
Lump
hard
No No
F7 Burlywood
Lump
hard
No No
F8 Ivory
Lump
hard
No No
F9 Burlywood
Lump
hard
No No
F10 Ivory
Rough
powder
No No
F11 Ivory
Rough
powder
No No
F12 Ivory
Rough
powder
No No
F13 White
Rough
powder
No No
F14 Ivory
Rough
powder
No No
3.2 Degree of Substitution Analysis
(DS)
The maximum degree of substitution for CMC is 3
with an average value between 0.4 to 1.5. The higher
degree of substitution of CMC, the more increase
solubility in water (Aambjornsson, 2015). The
results of DS analysis of CMC which have been
synthesized are shown in Table 4.
As it is known, using a mixed of solvents in
various composition ratio could affect the value of
DS. From the table 4 below, it can be seen that CMC
were synthesized using isopropanol solvent mixture
has a higher DS compared to using a solvent mixture
of benzene. It can be explained by the increasing
composition of the solvent mixture of isopropanol,
the rising of the polarity of a solution (Kalem and
Johangir, 2007; Pitaloka, 2017).
Synthesis and Characterization of Carboxymethyl Cellulose using Solvents Variations
1017

Table 4: Degree of Substitution of CMC.
Formula Solvents DS
Isopropanol: N-Butanol
F1 80:20
0.523
F2 50.50
0.522
F3 30:70
0.561
Benzene:Ethanol:Water
F4 70:20:10
0.412
F5 50:30:20
0.382
F6 50:50:0
0.751
Benzene:Isopropanol
F7 30:70
0.694
F8 50:50
0.628
F9 70:30
0.623
Isopropanol:Isobutanol
F10 70:30
0.797
F11 50:50
0.890
F12 30:70
0.757
Isopropanol:Ethanol: Water
F13 70:20:10
0.887
F14 50:50:0
0.906
Microcrystalline cellulose was used as the main
ingredient of CMC manufacture had polar
properties, wherein the cellulose will be easier to
expand with more polar solvents. In addition, the use
of isopropanol during synthesis CMC was known to
produce fewer side reactions such as sodium
glycolate (Im, 2018).
Based on Table 2, the polarity index of water
was higher than ethanol, but the value of DS
decreased with increasing the ratio of water used in
the solvent mixture. This is due to the small
molecular weight of water, while the
microcrystalline cellulose has a large molecular
weight. So that cellulose can not mix with the water
because of differences in molecular weight which
quite large
.
At high water content also maked the cellulose
was more decomposed by alkali and could damage
the destruction of the cellulose crystal structure,
which inhibits the diffusion of small reagent
molecules into it. It caused more NaOH
and NaMCA were remained in the solvent. In
addition, the CMC product thus obtained had fewer
degree of substitution and could be easily
decomposed by alkali. The solvent with high water
content were more polar than low moisture
content. These factors lead to more side reactions,
thus decreasing the availability of NaMCA and
the degree of substitution for CMC (Zhang,
1993). This applies also to the mixture of
isopropanol mixed solvents or benzene mixed
solvents having a lower DS value compared to a
mixture of waterless solvents.
The synthesized CMC using a solvent mixture of
isobutanol obtained a higher DS value than using a
solvent mixture of n-butanol. Although the polarity
index value of isobutanol and n-butanol is the same,
but the solvent mixture is influenced by the
composition of its chemical structure.
In this study, using a solvent with a mixture of
benzene and isopropanol was obtained a higher DS
value compared with a mixture of benzene and
ethanol. This shows that the solvent mixture has a
high polarity index difference will cause a decrease
in the value of DS. According to Zhao (2003)
benzene would adjust the polarity of the solvent
system. The lower polarity was achieved by
increased the percentage of benzene, which tends to
decreased solvent accessibility and NaOH to the
cellulose chain. The CMC structure showed the
crystal structure of the CMC synthesized with
benzene completely destroyed and the CMC chain is
cleary expands by the solvent.
Of the various types and compositions of solvent
were used in this study, the maximum DS value was
obtained on Isopropanol: Ethanol solvents (50:50),
by 0.9. Increased accessibility of solvents and
etherification reactants into the cellulose can
increase of the rate of reaction, thereby increasing
the DS and viscosity of the CMC solution.
When the solvent contains high levels of
alcohols, cellulose will be more easily alkalized. The
alkalized cellulose possesses less crystalline
aggregation. This causes the reaction to be faster and
to result a higher DS value. The molecular weight of
the product has a relationship to alcohol content as
well as to the other caracteristics (Zhang, et, al,
1993).
On the other hand, the microcrystalline cellulose
has a smaller particle size so it has a large surface
area. This will affect cellulose swelling in the
solvent, which will affect the quality of the CMC
produced.
3.3 Analysis of FTIR
FTIR analysis is carried out by comparing the
commercial microcrystalline cellulose and CMC
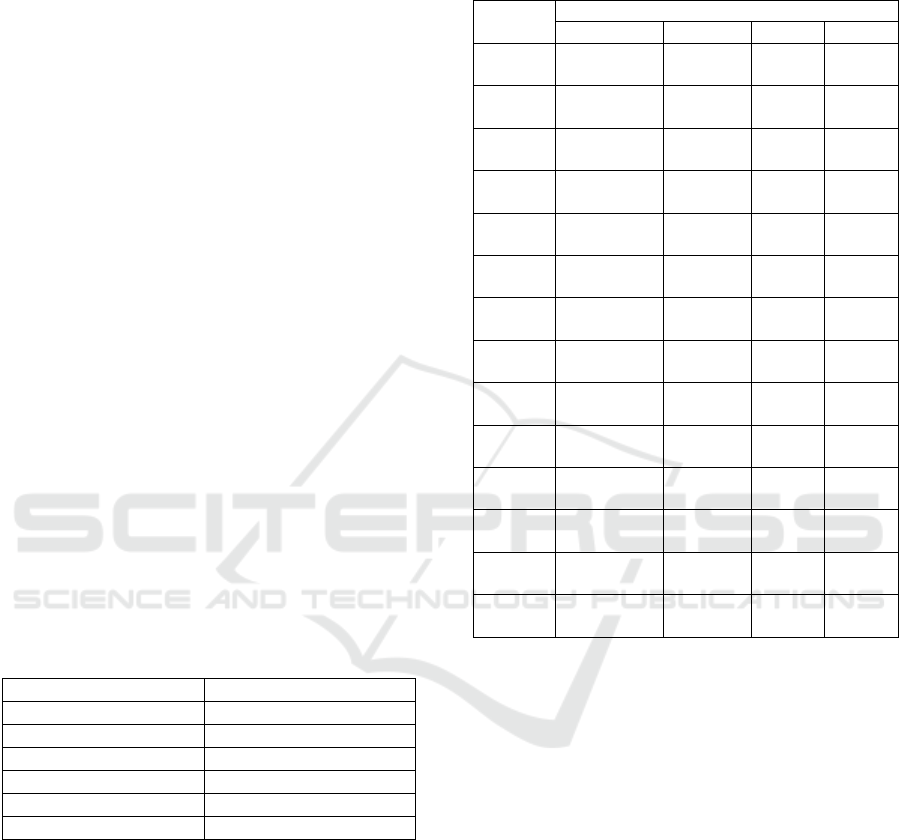
which can be seen in Figure 1. From the figure can
be seen the differences between the spectrum of
microcrystalline cellulose and carboxymethyl
cellulose. In the area of wave number 3000 – 2850
cm
-1
and 1200-1000 cm
-1
, a commercial CMC had a
slighter spectra than microcrystalline cellulose.
ICOSTEERR 2018 - International Conference of Science, Technology, Engineering, Environmental and Ramification Researches
1018
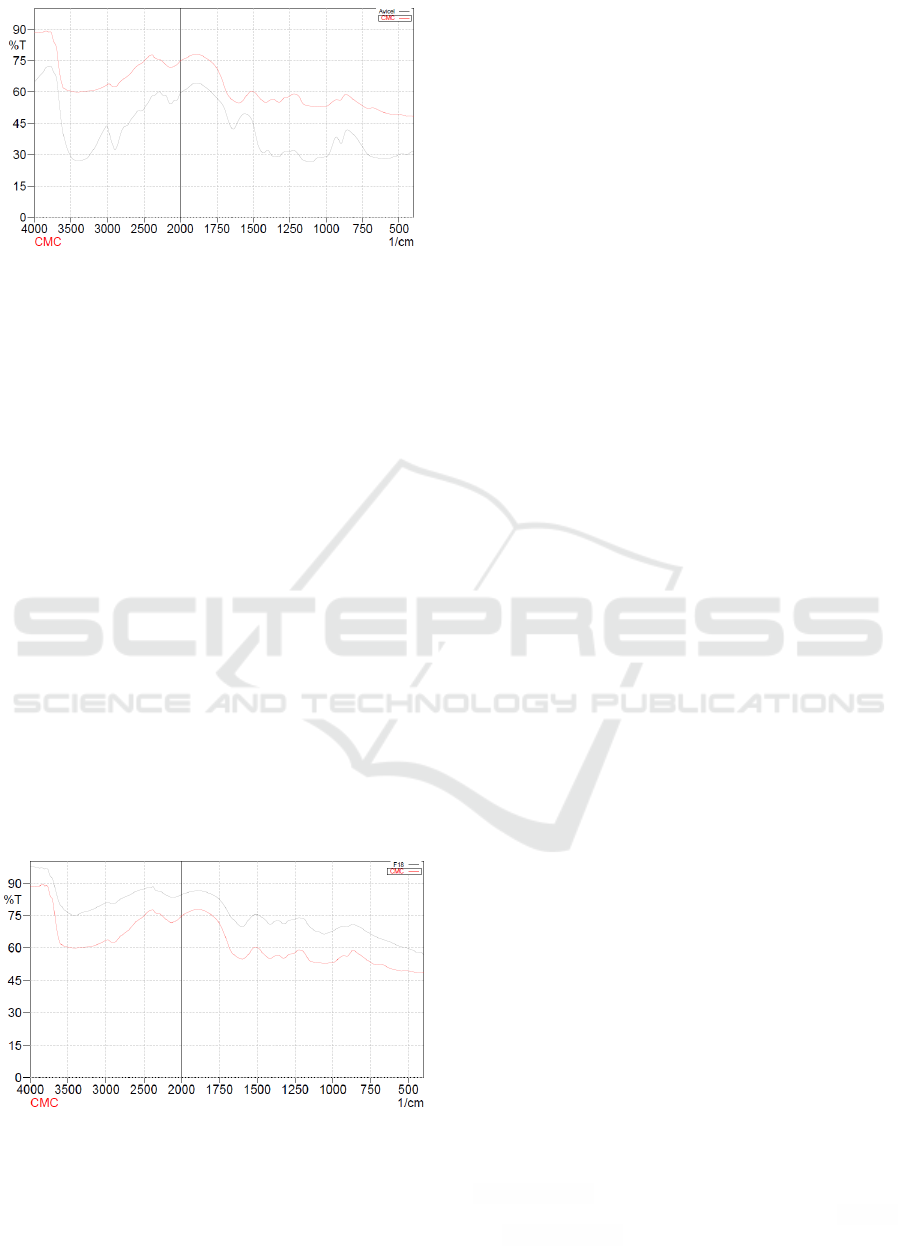
Figure 1: IR spectrum between CMC commercial and
microcrystal cellulose.
In addition, FTIR analysis of synthesized CMC
which had a maximum DS value with solvent
Isopropanol: Ethanol (50:50) is shown in Figure 2. It
indicated some point absorption at 1600.92 cm
-1
and
1415.75 cm
-1
. The highlights of the spectrum at a
wave number of 1600.92 cm
-1
with strong
absorption indicates the presence of carbonyl group
(COO), and at 1415.75 cm
-1
indicates methyl (-CH
2
).
It demonstrated a carboxymethyl had been
substituted in structure of the synthesized Na-CMC.
Carboxyl group as salt structures had a range of
waves ranging between 1600-1640 cm
-1
to 1400 to
1450 cm
-1
.
Furthermore, based on the analysis of FTIR of
synthesized CMC were obtained the stretching
vibration in some wave numbers, namely at 3421.72
cm
-1
indicated the -OH group, 2893.22 cm
-1
to
2927.94 cm
-1
showed -CH aliphatic. Figure 2
illustrated the comparison of the infrared spectrum
of commercial CMC and synthesized CMC. It
showed similar spectrum and functional groups in
both of them.
Figure 2: IR spectrum between commercial CMC and
synthesized CMC by Isopropanol: Ethanol (50:50)
4 CONCLUSIONS
Quality of the synthesized CMC is affected by the
solvent used. This study found that synthesis CMC
using mixed solvents content of water and solvent
with high polarity distinction would produce CMC
with a low degree of substitution values. In this
work, CMC was successfully synthesized using the
best solvent consisting of a mixture of Isopropanol:
Ethanol (50:50) with a DS value of 0.9 on the
addition of 10% NaOH at 55
o
C for 3 hours, and 4
grams NaMCA.
ACKNOWLEDGEMENTS
The authors gratefully acknowledge that the present
research is supported by Universitas Sumatera Utara.
The support is under the research grant TALENTA
USU of Year 2018 Contract Number
2590/UN.5.1.R/PPM/2017 on 16
th
March 2018
REFERENCES
Aambjornsson, H., A., Schenzel, K., Germgard, U., 2015.
Carboxymethyl cellulose produced at different
mercerization condition and characterized by NIR FT
Raman spectroscopy in combination with multivariate
analytical methods. Bioresources. 8. 1918-1932.
Cash, M., J., Caputo, S., J., 2010. Cellulose derivatives. In
A Food stabilizer, thickener, and gelling agents.
Willey-Blackwell. United Kingdom. 94-115.
Heinze, T., Pfeiiffer, K., 1999. Studies on the synthesis
and characterization of carboxymethylcellulose. Die
Angewandte Makromolekulare Chemie. 266 (1), 37-
45.
Im, W., Lee, S., Abhari, A., R., Youn, H., J., Lee, H., L.,
2018. Optimization of carboxymethylation reaction as
a pretreatment for production of cellulose nanofibrils.
Cellulose. Springer Science+Business Media B V part
of Springer Nature.
Ismail, N., M., Bono, A., Valintinus, A., C., R., Nilus, S.,
Chng, L., M., 2010. Optimization of reaction condition
for preparing carboxymethyl cellulose. Journal of
Applied Sciences. 10 (21). 2530-2536.
Kamel, S., Jahangir, K., 2007. Optimization of
carboxymethylation of starch in organic solvent.
International Journal of Polymeric Materials. 56. 511-
519.
Koh, M., H., 2013. Preparation and characterization of
carboxymethyl cellulose from sugarcane
bagasse (Doctoral dissertation, UTAR).
Maida, J., 2017. Global Carboxymethyl Cellulose Market -
Drivers and Forecasts by Technavio (London:
Bussiness Wire).
Synthesis and Characterization of Carboxymethyl Cellulose using Solvents Variations
1019

Mulyatno, H., A., Pratama, O., I., Inayati, I., 2017.
Synthesis of carboxymethyl cellulose (CMC) from
banana tree stem: influence of ratio of cellulose with
sodium chloroacetate to properties of carboxymethyl
cellulose EQUILIBRIUM Journal of Chemical
Engineering. 16 (2).
Musfiroh, I., Hasanah, A., N., Budiman, I., 2013. The
Optimmization of sodium carboxymethyl cellulose
(NaCMC) synthesized from water hyacinth
(Eichhornia crassipes (Mart.) Solm) cellulose.
RJPBCS. 4. 1092-1099.
Pitaloka, A., B., Saputra, A., H., Nasikin, M., 2017. The
effect of isopropyl alcohol-2-butanol mixed solvent on
degree of substitution of carboxymethl cellulose from
water hyacinth (Eichhornia crassipes) cellulose.
International Journal of Applied Engineering
Research. 12 (22). 12546-12553.
Safitri, D., Rahim, E., A., Prismawiryanti, P., Sikanna, R.,
2017. Synthesis of carboxymethyl cellulose (CMC) of
durian peel (Durio zibethinus) cellulose. KOVALEN.
3. 58-68.
Singh, R., K., Khatri, O., P., 2012. A scanning
electronmicrocope based new method for determining
degree of subttution of sodium carboxymethy
cellulose. Journal of Microscopy. 246 (1). 43–52
Stigsson, V., Kloow, G., Germga, U., 2006. The influence
of the solvent system used duringmanufacturing of
CMC. Cellulose. 13 (6). 705 -712.
Zhang, J., Li, D., Hong, X., Yu, Z., Shi, Q., 1993. Solvent
effect on carboxymethylation of cellulose. Journal of
Applied Polymer Science 49. 741-746.
Zhao, H., Cheng, F., Li, G., Zhang, J., 2003. Optimization
of a process for carboxymethyl cellulose (CMC)
preparation in mixed solvents. International Journal of
Polymeric Materials. 52. 749-759.
ICOSTEERR 2018 - International Conference of Science, Technology, Engineering, Environmental and Ramification Researches
1020
