
The Influence of the Ethanol Extracts of Numerous Plants on the
Development and Efficiency of the Nourishment Intake of the Fifth
Instar Larvae of Heliothis Armigera Hubner
Nursal
1
, and S. Ilyas
1
1
Faculty of Mathematics and Natural Science, Universitas Sumatera Utara, Jl. Bioteknologi No 1, Medan, Indonesia
Keywords: Botanical Insectisides, Heliothis Armigera Hubner.
Abstract: A study has been conducted on the impact of ethanol extract from various plants of Sweet orange peel
(Citrus sinensis), Mexican Sunflower (Tithonia diversifolia), Ginger rhizome (Zingiber officinale), and
Lemongrass (Cymbopogon citratus) on the development and efficiency of diet utilization of fifth instar
larvae of H. Armigera. The research implemented the Complete Randomized Designed (CRD) using the
treatment concentrations of (0,00%, 0.25%, 0,50%, 1,00%, 2,00%, 4.00%) and iteration of fifteen larvae.
The observation data were analyzed by variance if there were differences then followed by DnMRt test of
5%. The results showed that all treatment concentrations could reduce growth; Relative Growth Rate (RGR)
and efficiency of diet utilization, i.e., relative consumption rate (RCR), Efficiency of conversion of ingested
diet (ECI), Efficiency of conversion of the digestive nourishment (ECD). On contrary, there is an increase in
the Approximate Digestibility (AD) value, and statistically, the control is varied as the treatment
concentrations of 1.00% - 4.00% for (RCR, RGR, AD) and the treatment concentrations 0.50% of to 4.00%
set for (ECI, ECD). Overall, the effective concentration that affects the growth rate and the efficiency of
food consumption of A. armigera larvae is at a concentration of 2.00%.
1 INTRODUCTION
In an effort to control plant pests, the high frequency
usage of synthetic insecticides and arbitrary
application of the insecticides will result in
tremendous negative impacts such as resistance and
resurgence, killing of useful organisms,
environmental pollution, and insecticide residues
that are very harmful to human health because it can
cause cancer, kidney damage, genetic mutations,
many more (Harborne, 1987). The high residual
content can also weaken the selling value of the
vegetable commodities, primarily for export
purposes since vegetables with a residual content
above the threshold will be rejected by the importing
country, so it is very detrimental to the economic
sector (Grainge and Ahmed, 1988).
For this reason, the government is keen to
socialize how significant the impact of the loss of
synthetic insecticide use, with the hope that the
farmers as the producers are expected to fulfill these
demands, including conducting organic farming,
because in organic farming pest control must be
based on plant insecticides. The government
recommends the organic pesticides due to its nature,
including biodegradable, selectivity (relatively safe
against natural enemies of the pest), compatibility
(can be combined with other pest control
components), can slow down the resistance rate, and
ensure resilience and sustainability in farming
(Rattan, 2010).
In Tanah Karo, horticultural farmers use
botanical insecticides from several types of plants to
control pests in organic farming systems, including:
sweet orange peel (C. sinensis), Mexican sunflower
(T. diversifolia), ginger rhizome (Z. officinale), and
lemongrass (C. citratus) that have potential as
botanical insecticides (Harborne, 1987).
Tthe use of plant-based insecticides by farmers
in Tanah Karo must be examined to attract Tanah
Karo farmers to work with the organic farming
system. The evaluation was performed by searching
for secondary metabolites, the concentration of
ethanol extract from the organic insecticides of
sweet orange peel (C. sinensis), Mexican sunflower
(T. diversifolia), ginger rhizome (Z. officinale), and
1082
Nursal, . and Ilyas, S.
The Influence of the Ethanol Extracts of Numerous Plants on the Development and Efficiency of the Nourishment Intake of the Fifth Instar Larvae of Heliothis Armigera Hubner.
DOI: 10.5220/0010102510821086
In Proceedings of the International Conference of Science, Technology, Engineering, Environmental and Ramification Researches (ICOSTEERR 2018) - Research in Industry 4.0, pages
1082-1086
ISBN: 978-989-758-449-7
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

(1)
(3)
(4)
(5)
(2)
lemongrass (C. citratus) on the growth and
efficiency of food consumption of pest insects, as for
this research is 5th Instar Maggot of H. armigera.
The usage of botanical insecticides in
overcoming the pests of H. armigera issues has been
widely carried out. This is due to its very destructive
nature in many horticultural plants and its high
resistance to insecticides, so the concentration of
botanical insecticides that can cause death in the pest
insects will be more effective and efficient in
controlling other horticultural pests.
2 METHODOLOGY
2.1 Maintenance of Experimental
Animals
The larvae from the cornfield are nurtured in a
laboratory with its natural food. The mature larvae
are inserted into the breeding cage. Inside the cage
was placed a solution of honey and sugar as food,
and on the top and three sides of the cage were
placed clothes as a place for laying the eggs. After
the eggs hatched, the larvae are kept in artificial
food in plastic cups to have relatively the same size
until it reached the fifth instar larvae as the test
animals (Waldbauer et all, 1984).
2.2 Provision of the Artificial Food
800 ml of distilled water were boiled to dissolve
other ingredients. Mix 50 grams of corn meal, 50
grams of soybean juice and 30 grams of wheat germ,
then blend with distilled water. Then, mix 20 grams
of rice flour, 50 grams of cornstarch, 50 g of sugar
and 100 ccs of distilled water. After becoming a
paste, add 12 grams of vitamin, 6 grams of ascorbic
acid, , 2.5 grams of nipagine, and 2 grams of sorbic
acidthen beat well. Next enter ten ccs of corn oil.
After the temperature is 70 ° C, all the ingredients
were mixed. Add 15 grams of yeast and 10 ml of
formalin. Furthermore, the food was placed on the
plastic cups where the larvae were maintained.
2.3 Procurement of the Ethanol
Extract of Sweet Orange Peel
(C. sinensis), Mexican Sunflower
(T. diversifolia), Ginger Rhizome
(Z. officinale), and Lemongrass
(C. citratus)
Sweet orange peel (C. sinensis), Mexican sunflower
(T. diversifolia), ginger rhizome (Z. officinale), and
lemongrass (C. citratus) were macerated with
ethanol for 3x24 hours. The process was repeated
until the color of the obtained solution is translucent
(assumed ethanol attracted all polar and non-polar
compounds). The obtained macerate produced a
concentrated ethanol extract of sweet orange peel
(C. sinensis), Mexican sunflower (T. diversifolia),
ginger rhizome (Z. officinale), and lemongrass (C.
citratus) since it was condensed at 40 ° C with a
rotary evaporator (Isman, 2008).
2.4 Experimental Test of the Ethanol
Extract of Sweet Orange Peel
(C. sinensis), Mexican Sunflower
(T. diversifolia), Ginger Rhizome
(Z. officinale), and Lemongrass
(C. citratus) on the Development
and Efficiency of the Nourishment
Intake of the Fifth Instar Larvae of
H. armigera
The artificial food was prepared with 6 treatment
concentration types of (0%, 0.25%, 0.50%, 1.00%,
2.00%, 4.00%) with Nutrition Index testing
parameters (Waldbauer, 1968):
Relative Growth Rate (RGR)
Relative Consumption Rate (RCR)
The efficiency of Conversion of Digested
Food (ECD)
The efficiency of Conversion of Ingested
Food (ECI)
Approximate Digestibility (AD)
The Influence of the Ethanol Extracts of Numerous Plants on the Development and Efficiency of the Nourishment Intake of the Fifth Instar
Larvae of Heliothis Armigera Hubner
1083

Note: G = Larvae weight gain during meal period
(initial weight of larvae - final weight of larvae)
F = Amount of the food depleted
E = Dry weight of feces
T = Eating period
A = The average weight of grub during the feeding
period (initial weight of larvae + finale weight of
larvae 2)
2.5 Experimental Design
This study applied the Complete Randomized
Design (CRD) with six mixture concentration of
treatments (0%, 0,25%, 0,50%, 1,00%, 2,00%,
4,00%) with a 1:1 ratio of the ethanol extract from
sweet orange peel (C. sinensis), Mexican sunflower
(T. diversifolia), ginger rhizome (Z. officinale), and
lemongrass (C. citratus) and a recurrence of fifteen
larvae.
3 DATA ANALYSIS
Variance analyzed each observation parameter data,
if there were significant differences, it followed by
the Duncan test at a 5% of confidence level.
4 RESULT AND DISCUSSION
In accordance with the test on The Influence of
Ethanol Extracts of Numerous Plant Types on The
Development and Efficiency of Nourishment Intake
of fifth Instar Larvae of Heliothis Armigera Hubner,
the obtained results are as follows:
4.1 The Impact of the Ethanol Extract
of Sweet Orange Peel (C. sinensis),
Mexican Sunflower
(T. diversifolia), Ginger Rhizome
(Z. officinale), and Lemongrass
(C. citratus) on Relative Growth
Rate (RGR) and Relative
Consumption Rate (RCR) of the
Fifth Instar Larvae of H. armigera
Based on the performed test, as in Table 1, it shows
that all treatment concentration of the ethanol
extracts of sweet orange peel (C. sinensis), Mexican
sunflower (T. diversifolia), ginger rhizome (Z.
officinale), and lemongrass (C. citratus) can lead to
the depression of Relative Growth Rate (RGR) and
Relative Consumption Rate (RCR).
Table 1: Relative Growth Rate (RGR) and Relative
Consumption Rate (RCR) of the fifth instar larvae of H.
Armigera on the food with the sweet orange peel’s ethanol
extracts (C. sinensis), Mexican sunflower (T. diversifolia),
ginger rhizome (Z. officinale), and lemongrass (C.
citratus).
Treatment
(%)
RCR
(mg/mg/h)
RGR(mg/mg/h)
0,00 1,50a ± 0,04 0,27a ± 0,01
0,25 1,57b ± 0,07 0,27a ± 0,01
0,50 1,52a ± 0,08 0,25ab ± 0,01
1,00 1,43b ± 0,04 0,23bc ± 0,02
2,00 1,20d ± 0,09 0,19cd ± 0,01
4,00 1,07e ± 0,11 0,15d ± 0,02
Notes: N=15 for every treatment. The mean
value of ± SE (error) followed by the same
lowercase letter in one column is not
significantly different (Duncan test will be
performed after ANOVA at 5% level)
Table 1 shows that in the treatment of 0.25%,
there was a 4.46% increase in RCR with a value of
1.57 mg/mg / h which is statistically different
compared to the control RCR value of 1.50 mg/mg /
h. This implies that the treatment concentrations
have not affected the larvae feeding activity. The
decreased value of RCR, RGR began to occur at the
concentration of 1.00% - 4.00%, and it decreases as
the treatment concentration increased. This decrease
can be caused by the ethanol extracts of sweet
orange peel (C. sinensis), Mexican sunflower (T.
diversifolia), ginger rhizome (Z. officinale), and
lemongrass (C. citratus) which contain secondary
metabolites that are toxic to the larvae. In previous
research, it stated that the plants of Azadirachata
indica, Curcuma longa, Acorus calamus (Rajput et
all, 2003), Azadirachata indica, Quassina amara
(Aggarwal et all, 2006), Cuorophia guianensis
(Dadang and Djokol, 2011), Mahogany, Neem,
Tobacco (Rahman et all, 2014), Nigella sativa plant,
Aristolochia, and Jatropha curcas (Baskar et all,
2010) are toxic to H. armigera. The combination of
sweet orange peel with neem leaves is toxic to
Spodoptera litura (Tarigan et all, 2012). It is shown
that the secondary metabolites contained in these
four types of plants can be toxic to insects. The
plants can produce various types of secondary
metabolites such as flavonoids, terpenoids,
alkaloids, and more, which are used as self-defense,
and are toxic to insects. Therefore plants can be used
as botanical insecticides (Rattan, 2010).
Table 1 also indicates that the decrement in RCR
will cause a decrease in larvae RGR. This decrease
ICOSTEERR 2018 - International Conference of Science, Technology, Engineering, Environmental and Ramification Researches
1084
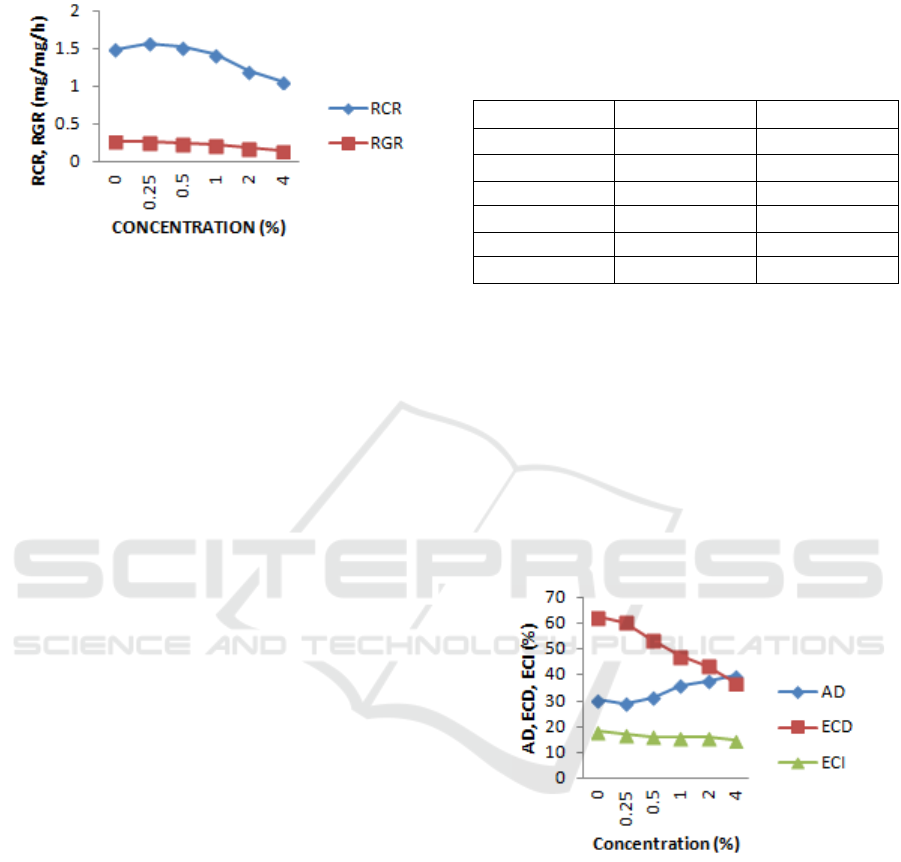
is greater as treatment concentration increases. The
details can be seen in Figure 1.
Figure 1: Relative Growth Rate (RGR) and Relative
Consumption Rate (RCR) of the fifth instar larvae of H.
Armigera on the food with the ethanol extracts of Mexican
sunflower (T. diversifolia), sweet orange peel (C.
sinensis), ginger rhizome (Z. officinale), and lemongrass
(C. citratus).
4.2 The Influence of the Ethanol
Extract of Sweet Orange Peel
(C. sinensis), Mexican Sunflower
(T. diversifolia), Ginger Rhizome
(Z. officinale), and Lemongrass
(C. citratus) on Approximate
Digestibility (AD), Efficiency of
Conversion of Ingested diet (ECI),
and Efficiency of Conversion of
Digested Food (ECD) of the Fifth
Instar Larvae of H. armigera
Based on the conducted test as shown in Table 2, it
shows that all treatment concentrations of the
ethanol extracts of sweet orange peel (C. sinensis),
Mexican sunflower (T. diversifolia), ginger rhizome
(Z. officinale), and lemongrass (C. citratus) that
were put into the larvae diet seem to affect the
efficiency of food use, namely; in the form of a
decrease in the efficiency of the conversion of
digested nutrient (ECD), as well as in the efficiency
of conversion of ingested food (ECI), also an
increase in the approximate digestibility (AD).
Table 2: Approximate digestibility (AD), the efficiency of
the conversion of digested nutrient (ECD), and the
efficiency of conversion of ingested diet (ECI) of the fifth
instar larvae.of H. armigera pada on the food added with
the ethanol extracts of sweet orange peels (C. sinensis),
mexican sunflower (T. diversifolia), ginger rhizome (Z.
officinale), and lemongrass (C. citratus).
Treatment(%) AD (%) ECD (%)
0,00 30,34a ± 1,53 62,63a ± 3,38
0,25 28,78a ± 0,98 60,37ab ± 3,10
0,50 31,01a ± 1,39 53,51bc ± 1,89
1,00 35,92b ± 2,05 47,21cd ± 3,45
2,00 37,41b ± 1,44 43,63de ± 3,16
4,00 39,71b ± 2,13 36,92e ± 2,23
Notes: N=15 for every treatment. The mean value of ± se
(error) followed by the same lowercase letter in one
column is not significantly different (Duncan test will be
performed after ANOVA at 5% level)
The digression of ECD and ECI occured in
treatment concentrations of 0,50% - 4,00% with a
decrease range of 14,56% - 41,05% and 11,67% -
19,78%) for ECD and ECI respectively. On the
contrary, the value of AD arisen at the treatment
concentrations of 1,00% - 4,00% with an increase of
18,39%-30,88%. The details of the test is shown in
Figure 2.
Figure 2: Approximate digestibility (AD), the efficiency of
conversion of ingested nourishment (ECI), and the
efficiency of the conversion of digested nourishment
(ECD) of the fifth instar larvae.of H. armigera pada on the
food added with the ethanol extracts of Mexican sunflower
(T. diversifolia), ginger rhizome (Z. officinale), sweet
orange peel (C. sinensis), and lemongrass (C. citratus).
The decrement of ECD and ECI, as well as the
increasing value of AD in treatment concentration of
0,50% - 4.0%, becomes higher as the treatment
concentration increases, and it differs from the
control value statistically. Thus it can be stated that
these treatment concentrations are effective to affect
The Influence of the Ethanol Extracts of Numerous Plants on the Development and Efficiency of the Nourishment Intake of the Fifth Instar
Larvae of Heliothis Armigera Hubner
1085

the efficiency of larvae food utilization. As an
indication, it can be seen from RGR value which is
also decreasing. Whereas the decrease in larvae
RGR will be greater as the ECD and ECI decrease.
While an increase in the value of AD is a
compensation response so larvae can survive.
According to the previous study, if there are toxic
compounds in the food, insects will perform
compensatory responses, including an increase in
AD values (Simpson and Simpson, 1990). Chaniago
et all (2013) explained the AD value of silkworm
will increase if there is a lack of nutrition in the
food. Furthermore, mangrove bark (Rhizophora
mucronata) is reported to be toxic and inhibits the
growth and feeding activity of H. armigera larvae
(Rajput et all, 2003). The combination of Annona
scuamosa seeds, Piper retrofractum fruit, Tephrosia
vogelii leaves is lethal and suppress the appetite of
the vegetable pests of Crosidolomia pavonang
(Grainge and Ahmed, 1988).
5 CONCLUSIONS
Based on the studies, it can be concluded that the
ethanol extracts of sweet orange peel (), Mexican
sunflower (T. diversifolia), ginger rhizome (Z.
officinale), and lemongrass (C. citratus) have effects
on the development and efficiency of diet intake of
the fifth instar larvae, such as the decline in
Efficiency of conversion of ingested nourishment
(ECI), Efficiency of conversion of digestive diet
(ECD), and relative consumption rate (RCR), as well
as value increment of the approximate digestibility
(AD) with the effective concentration of treatment at
2.0%.
ACKNOWLEDGEMENTS
Authors would like to thank the Rector of
Universitas Sumatera Utara for funding this research
through the program of Talent Basic Research 2018
(Contract Number: 246/UN5.2.3.1/PPM/KP-
TALENTA USU/2018), so authors can participate in
ICOSTEERR Seminar held on August 30-31, 2018.
REFERENCES
Aggarwal, N., Markus, H., and Thies, B. 2006. Evaluation
of bio-rational insecticides to control Helicoverpa
armigera (Hübner) and Spodoptera exigua (Hübner)
(Lepidoptera: Noctuidae) fed on Vicia faba L. MITT.
DTSCH. GES. ALLG. ANGEW. ENT. 15.
Baskar, K., Maheswaran, R., Kingsley, S., and
Ignacimuthu, S. 2010. Bioefficacy of Couroupita
guianensis (Aubl) against Helicoverpa armigera
(Hub.) (Lepidoptera: Noctuidae) larvae. Spanish
Journal of Agricultural Research , Volume 8(1): 135-
141
Chaniago, M., Tanjung, M., Nursal. 2013. The effect of
the quality of mulberry leaves Morus multicaulis on
the nutritional index of Bombyx silkworm mori L.
(lepidoptera:Bombicidae). Journal of Saintia Biologi,
Volume 1(3):19-25.
Dadang, and Djoko, P. 2011. Technological development
of Botanical Insecticides Formulas to control the
vegetable pests to produce a better quality product.
Journal of Agricultural Indonesia, Volume 16(2): 100-
111
Grainge, M., and Ahmed, S. 1988. Handbook of the plant
with pest control properties. John Wiley & Sons.
United States: New York.
Harborne, J.B. 1987. Phytochemical methods guiding the
modern way of analyzing plant. Translated by Padma,
W. K., and Soediro, I. Bandung: ITB.
Isman, M.B. 2008. Perspective Botanical insecticides: for
richer, for poorer. Pest Manag Sci, Volume 64: 8– 11.
Rahman, A. K. M. Z., Haque, M. H., Alan, S. N., and
Mahmuddunabi, M. 2014. Efficacy of Botanicals
against Helicoverpa armigera Hubner in Tomato. The
agriculturist, Volume 12 (1): 131-139.
Rajput, A. A., Sarwar, M., Bux, M., and Tofique, M.
2003. Evaluation of synthetic and some plant origin
insecticides against Helicoverpa armigera Hubner on
chickpea. Pakistan Journal of Biological Sciences,
Volume 6: 496-499.
Rattan, R. S. 2010. Mechanism of action insecticidal
secondary metabolites of plant origin. Crop protection,
Volume 29(9): 913-920.
Scriber, J. M., and Slansky, F. Jr. 1981. The nutritional
ecology of immature insects. Ann.rev.entomol,
Volume 26: 183-211.
Simpson, S. J., and Simpson, C. L. 1990. The mechanism
of nutritional compensation by Phytophagous insects,
Insects plant interaction. Florida: C.R.C. Press. pp.
111-160.
Tarigan, R., Tarigan, M. U., and Oemry, S. 2012.
Effectiveness Test of Sweet Orange Peel Solution and
Neem Leaf Solution to Control Spodoptera Litura F.
(Lepidoptera: Noctuidae) on Mustard Plants. Online
Jurnal of Agroecotechnology, Volume 1(1): 172-182.
Waldbauer, G.P. 1968. The consumption and utilization of
food by insects. Adv. Insect Physiol, Volume 5: 229-
288.
Waldbauer, G. P., Cohen, R. W., and Friedman, S. 1984.
An improved procedure for laboratory rearing of the
Corn Earworm, Heliothis Zea
(Lepidoptera:Noctuidae). The great lake Entomologist,
Volume 17(2): 113-118.
Smith, J., 1998. The book
, The publishing company.
London, 2
nd
edition.
ICOSTEERR 2018 - International Conference of Science, Technology, Engineering, Environmental and Ramification Researches
1086
