
Optical Technology for Fibrotic Skin Changes Objectification in
Experimental Systemic Scleroderma
Yulia Chursinova
1
, Dmitriy Kulikov
1
, Dmitry Rogatkin
1
, Irina Raznitsyna
1, 2
,
Darya Mosalskaya
1
and Maksim Bobrov
1
1
Moscow Regional Research and Clinical Institute "MONIKI", 61/2, Shchepkina str.,
Moscow, RF, 129110, Russian Federation
2
National Research Nuclear University MEPhI, 31, Kashirskoe highway, Moscow, RF, 115409, Russian Federation
Keywords: Skin Fibrosis, Inflammation, Fluorescence, Saturation, Non-invasive, Diagnostics, in Vivo.
Abstract: Currently the examination of skin fibrosis is based on subjective non quantitative methods and requires
invasive procedures. Optical techniques abled to evaluate different quantitative parameters of ordered
tissues can be used to solve these problem. Measurements of endogenous fluorescence intensity, regional
tissue oxyhemoglobin saturation, and blood filling volume allowed to define the high endogenous
fluorescence intensity of porphyrin in skin fibrosis. Besides that, the decrease in oxygen intake parameters
together with the fluorescence intensity increase of collagen was determined. Consequently the optical
diagnostic techniques can become an effective method for skin fibrosis evaluation.
1 INTRODUCTION
Fibrosis of different organs and systems is one of
severe medical issues as it affects a significant
proportion of the human population (Wermuth,
2015; Rockey et al., 2015). It is the main
pathological process not only in such autoimmune
disorders as scleroderma, rheumatoid arthritis,
Crohn’s disease, ulcerative colitis, and systemic
lupus erythematosus (Wynn et al., 2011), but also in
liver, kidney, lung diseases and heart failure
(Bataller et al., 2005; Wynn, 2008).
Currently there are a lot of studies on fibrosis.
The pathogenesis of tissue fibrosis is considered to
be a dynamic and reversible process connected with
inflammation and hypoxia (Driskell et al., 2013;
Manresa et al, 2014). Nevertheless, the histological
examination still remains the reference method for
diagnosis, which invasiveness is the main
disadvantage as it impairs the examined tissues state
(Monstrey et al., 2008).
Fibrotic skin changes are the defining features of
all scleroderma forms (Gabrielli et al., 2009). The
degree and the rate of fibrosis progression correlate
with patients’ death rate (Clements et al., 2000;
Khanna et al., 2010). In clinical practice the
modified Rodnan skin score (mRSS) that measures
the skinfold thickness is widely used for skin
fibrosis evaluation. However, the information
received when using this method is rather subjective.
The application of mRSS is essentially limited as it
requires special knowledge and skills from
physicians. Subcutaneous fat changes developed in
some patients suffering from this disease can also
lead to diagnostic pitfall. Furthermore skin score is
insensitive to initial presentation of a disease that is
however clinically significant (Maurer et al., 2014).
Non-invasive methods of skin fibrosis diagnosis
such as ultrasound scan, elastography, confocal
microscopy, and optical coherence tomography are
still of limited use in routine clinical practice due to
the lack of the accurate criteria for fibrosis
assessment (Kang et al., 2014). The microvascular
damage dominates in the pathogenesis of skin
fibrosis. That causes endothelial cell activation
leading to the hypoxia and the increase in the
amount of inflammation triggers that starts
uncontrolled inflammation response. As a result of it
fibroblasts excessively differentiate into
myofibroblasts, responsible for extracellular matrix
synthesis, which major component is collagen
(Jinnin, 2010; Hinz B. et al., 2012; Ho et al., 2014).
The excess of collagen is known to be detected by
laser fluorescence spectroscopy as this substance
fluoresces under UV light (Smirnova et al., 2012).
194
Chursinova, Y., Kulikov, D., Rogatkin, D., Raznitsyna, I., Mosalskaya, D. and Bobrov, M.
Optical Technology for Fibrotic Skin Changes Objectification in Experimental Systemic Scleroderma.
DOI: 10.5220/0006633101940199
In Proceedings of the 11th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2018) - Volume 1: BIODEVICES, pages 194-199
ISBN: 978-989-758-277-6
Copyright © 2018 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
Fluorophores responsible for inflammation and
hypoxia can also be detected in red and green
spectrum range (Franco et al., 2016).
In modern medicine the development of the rapid
and non-invasive method enabled to give a
quantitative assessment of skin fibrosis is absolutely
necessary. In such a case optical techniques have a
diagnostic potential to become the basis for a
fundamentally new approach to fibrosis
comprehensive assessment.
The aim of our study was to examine diagnostic
capabilities of optical technologies in animal skin
fibrosis evaluation. Animal models are still of a
great importance for skin fibrosis pathogenesis
investigation, and the results obtained can either be
reproduced in clinical researches or give a
meaningful data for understanding the pathogenesis
of this process (Avouac et al., 2013; De Langhe,
2015).
2 MATERIALS AND METHODS
The study was performed on the outbred white male
mice aged 6 weeks with a mass of 25-30 grams, N =
47. Animals were kept in vivarium standard
conditions in a 14 hour natural light at a temperature
of 21-23 °C and a humidity of 50 - 65%. They
received balanced granulated feed, that didn’t
include fluorophores and had a free water access.
The experiment was conducted in compliance
with the welfare of animals used in experiment
(Declaration of Helsinki), EU Directive 86/609/EEC
on the protection of animals used in experiments,
and European Convention for the Protection of
Vertebrate Animals Used for Experimental and other
Scientific Purposes (ETS 123) Strasbourg, 1986).
The relevant animal model of scleroderma was
used for fibrosis development. We chose bleomycin-
induced fibrosis as it allows to represent the initial
presentation of this disease (Avouac, 2014).
Animals were divided into two groups. In the
first one (N = 30) subcutaneous injections of
bleomycin (BLM) in a dose of 0,1 ml (100μL of
bleomycin preliminary dissolved in 0.9% NaCl,
concentration 0.5 mg/ml) were administered. In the
second one (control group, N = 17) subcutaneous
injections of 0,1 ml of 0,9 % NaCl (PBS) were
administered. All animals were daily injected in
shaved skin of interscapular region during 21 days.
The first four injections were made in different
angles of a marked square with a size of 1 cm², the
fifth one was done in its middle.
On the 0, 7, 14 and 21 day the endogenous
fluorescence intensity, regional tissue
oxyhemoglobin saturation, and blood filling volume
were measured in vivo, on the skin surface just
above the experimental area. All measurements were
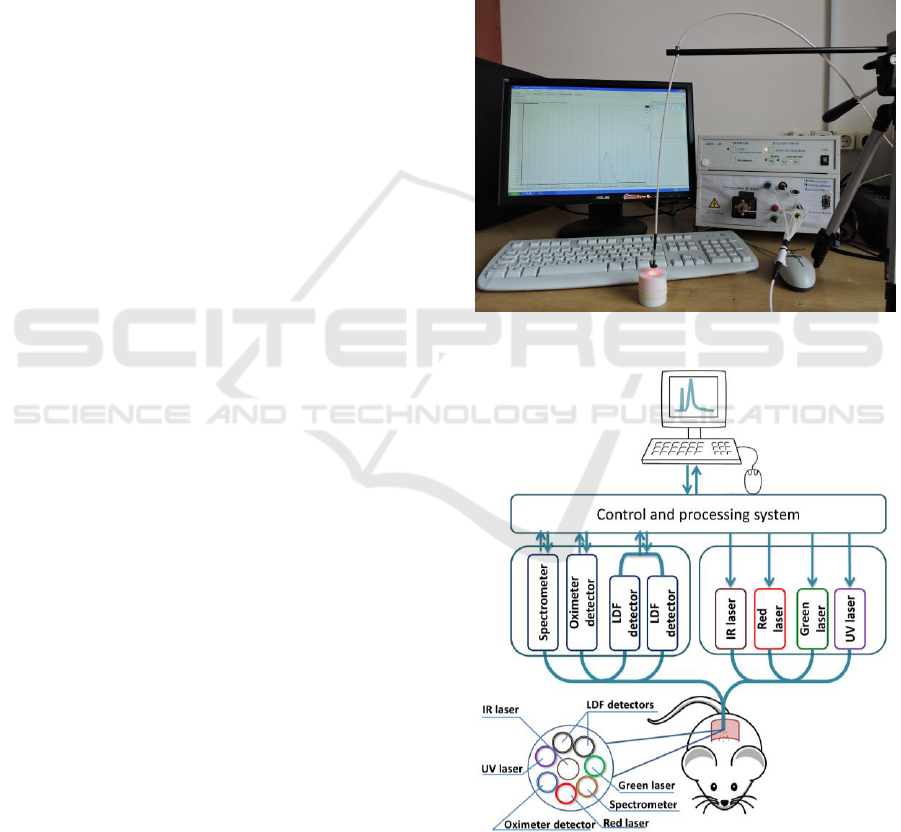
taken using non-invasive multifunctional laser
diagnostic system “LAKK-M” (SPE ‘LAZMA’ Ltd,
Russia) (Figure 1) on the operating regimes
“Fluorescence” and “Microcirculation” (Rogatkin et
al., 2009). Scheme of diagnostic system, the mouse
locations in the setup and sensor localization are
presented at figure 2.
Figure 1: Diagnostic system “LAKK-M”.
Figure 2: Scheme of the diagnostic system.
Low-power lasers with a wave length of λ
e
= 365,
535 and 635 nm were used for fluorescence
excitation. The output power at the distal end of the
Optical Technology for Fibrotic Skin Changes Objectification in Experimental Systemic Scleroderma
195
fiber-optical probe was about 2 – 3 mW for each
light source. The wavelengths on which the
fluorescence had a maximum value were marked
with λ
f
. Thus for collagen λ
f
= 455 nm, for porphyrin
λ
f
= 610 nm. It should be noted, that it is hard to
separate the fluorescence of collagen and elastin, so
in the following we considered that the fluorescence
on the wave length of λ
f
= 455 nm represents both of
fluorophores. In this study the intensity dynamics on
this wavelength (later on – “the fluorescence
intensity”) in controlled equivalent intensity of
irradiation was evaluated.
In “Microcirculation” operating regime laser
Doppler flowmetry and tissue reflectance oximetry
were used enabling to continuously register tissue
saturation and blood filling volume values in
percent.
The relative oxygen consumption rate (U)
characterized by the oxygen intake per tissue blood
flow volume unit was assessed according to the
time-averaged (15 s) measurements using the
following formula (Rogatkin et al., 2013):
U= (S
p
O
2
- S
t
O
2
)/ V
b
(1)
S
t
O
2
, means tissue oxyhemoglobin saturation, V
b
,
means blood filling volume. In this case, S
p
O
2
is the
functional pulse saturation of the oxyhemoglobin
fraction in the arterial peripheral blood. It was
assumed equal to 98%.
Histological samples were taken on 0, 7, 14, and
21 day. Skin fragments 1,0 cm 1,0 cm in size were
separated from the research region followed by
material examination according to a standard
protocol. An epidermis condition, inflammatory
changes in dermis, subcutaneous fat, and panniculus
carnosus, as well as dermis thickness and collagen
fibers structure were assessed.
Furthermore, a noncompetitive enzyme-linked
immunosorbent assay method was used to evaluate
C-reactive protein in control period. For this purpose
we used a mice blood serum having been
centrifuged in 1500 g mode during 15 minutes.
Researches were performed on microplate
photometer for enzyme immunoassay Stat Fax 2100,
Awareness Technology, USA. We also used Mouse
CRP (an enzyme immunoassay kit for the
quantitative measurement of mouse CRP), Czech
Republic.
Statistical analysis was carried out in Microsoft
Excel 2016. A hypothesis for the difference between
two groups was tested by the comparison of
arithmetic means and the construction of 95%
confidence intervals for them.
3 RESULTS AND DISCUSSION
In BLM animal group a skin fibrosis was reproduced
by the 21
st
day of experiment. The histological
pattern of tissue in the injection area in both groups
on the 21
st
day of experiment is shown in the Figure
3.
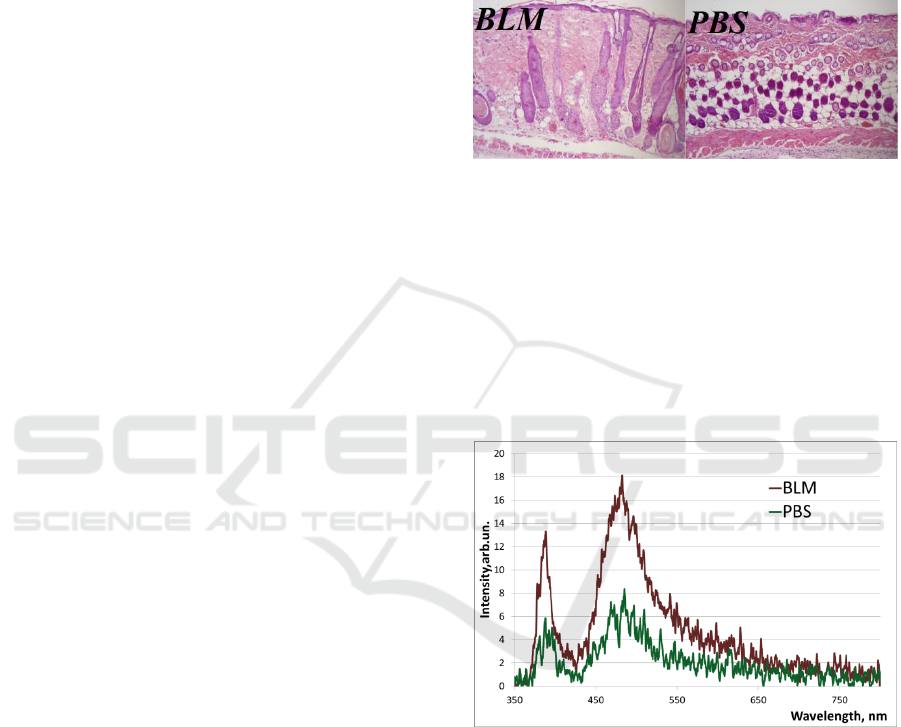
Figure 3: Skin histology on the 21
st
day, haematoxylin and
eosin stain at a magnification of x 100. In BLM group:
flatness of epidermis, thickened derma, homogenisation of
collagen fibres, hyperplasia of hair follicles, dermal
adipose layer depletion, inflammatory infiltrate under
panniculus carnosus. In PBS group: the structure of the
epidermis and dermis is unchanged, inflammatory
infiltrate under panniculus carnosus is detected.
Examples of measured fluorescence spectrum
from the injection area at λ
e
= 365 nm on the 21
st
day
of the experiment is shown in the Figure 4.
Figure 4: The example of the fluorescence spectrum in
BLM and PSB groups on the 21
st
day of the experiment; λ
e
= 365 nm.
Obtained spectra are characterized by the
presence of two maxima corresponding to the
backscattering peak (365 nm) and to the
fluorescence of the collagen and elastin (455 nm).
Figure 5 illustrates the results of optical
measurements and laboratory analysis.
BIODEVICES 2018 - 11th International Conference on Biomedical Electronics and Devices
196
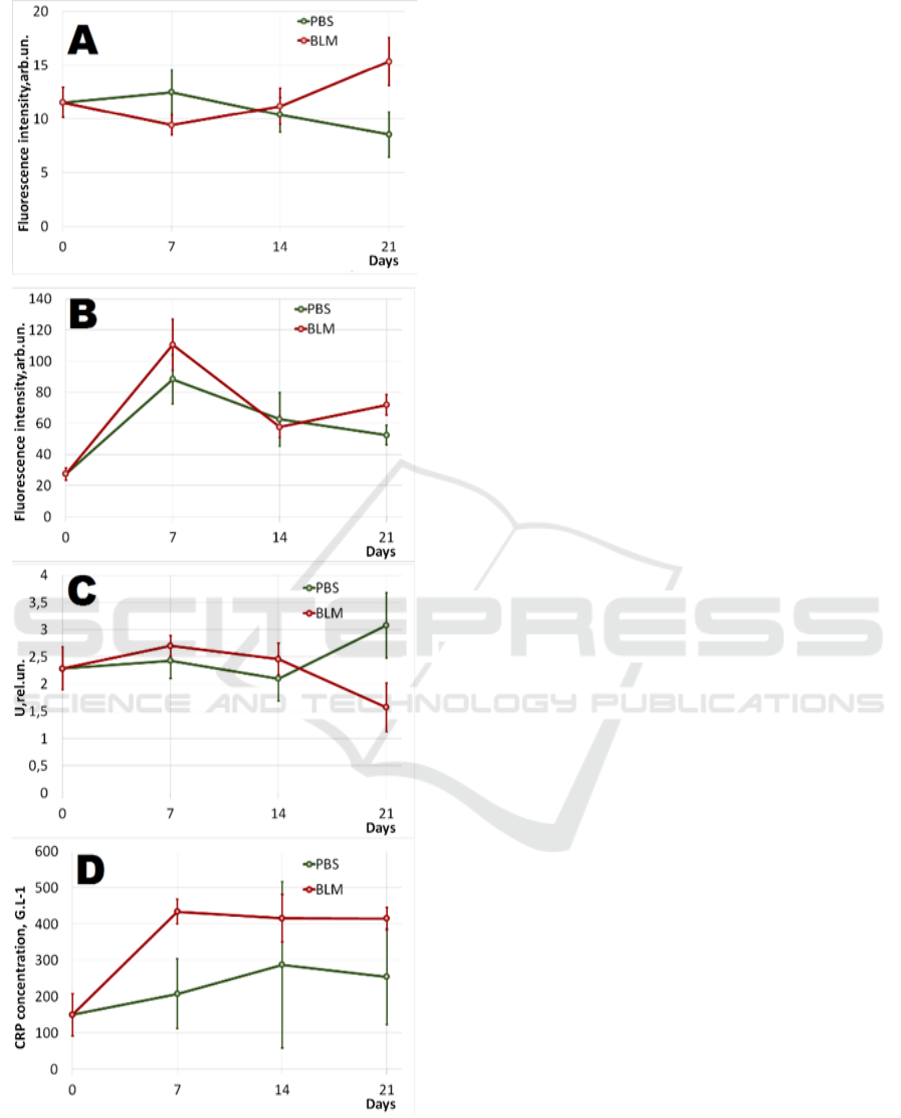
Figure 5: Dynamic by days: А. - Fluorescence intensity
rate of collagen and elastin (λ
e
= 365 nm, λ
f
= 455 nm); B.-
Fluorescence intensity rate of porphyrin (λ
e
= 535 nm, λ
f
=
610 nm); C. - Relative oxygen consumption rate in tissues;
D. - C-reactive protein rates. 95% confidence interval is
shown in the chart.
Figure 5A shows the dynamics of the
t6yrteaverage fluorescence intensity rate of collagen
and elastin in both groups. On the 21
st
day a
statistically significant difference in BLM and PBS
groups appeared.
The results of porphyrin fluorescence (Figure 5B)
demonstrate the increase in the average fluorescence
intensity as compared to the 0 day of the experiment
in both animal groups. Besides that, a statistically
significant difference in BLM and PBS groups were
received on the 21
st
day.
The results of relative oxygen consumption rate
demonstrate its statistically significant decrease by
the 21
st
day in BLM group (Figure 5C).
Figure 5D shows the increase in C-reactive
protein rate of animal blood serum in both groups.
Statistically significant differences in BLM and PBS
groups were assessed on the 7
th
day of experiment.
All data obtained during the experiment leads to
a number of conclusions and assumptions. Thus, we
believe, that the increase in endogenous fluorescence
intensity rate of collagen and elastin on the 21
st
day
in BLM group is due to its accumulation in the
histologically confirmed fibrosis area. Whereas
collagen is the main extracellular substance of
connective tissue in skin fibrosis (Ho et al., 2014),
we consider, that the impact of elastin fluorescence
is imperceptible.
Tissues in which collagen fibers are extensively
synthesized are known to have a high oxygen
requirement. Nevertheless, the formed fibrosis
decreases it due to the reduction in the number of
cell elements (Lokmic et al., 2012). The relative
oxygen consumption rate on the 21
st
day in BLM
group is representative of this causation. (Figure 5A
and 5C). Some researchers consider that fibrosis is
permanent when a tissue becomes few-celled and
has a lack of biologically active molecules essential
to the extracellular substance deterioration (Iredale,
2007; Wynn, 2008, Rockey, 2015). We believe that
contemporary examination of collagen fluorescence
and the relative oxygen consumption rate in tissues
will allow to establish the synchronous nature of this
process. The information received is essential to
clinicians as the data on the degree and the rate of
skin fibrosis progression in systemic scleroderma
enable to diagnose this form of disease, to determine
a management strategy in time, and to predict the
clinical course.
Porphyrins are well known to response on
metabolic changes in tissues quickly. Their synthesis
is particularly enhanced in cells during inflammation
and hypoxia (Petritskaya et al., 2015). Vasculopathy
is known to cause the oxygen delivery decrease in
Optical Technology for Fibrotic Skin Changes Objectification in Experimental Systemic Scleroderma
197

cells at the beginning of scleroderma. In the
following, fibrosis tissue induces perfusion defect
and becomes the main cause of hypoxia (Van Hal et
al, 2011). Since bleomycin-induced model better
represents tissue fibrotic changes (Yamamoto et al,
2011), we suppose that statistically significant
magnification in porphyrin fluorescence rate on the
21
st
day indicates a chronic hypoxia and is based on
the perfusion defect.
We also assumed the development of
inflammation of the dermis during fibrosis formation
in experimental model. High levels of blood serum
C-reactive protein in BLM group on the 7th day
were determined in support of it. That implies the
disease high activity and is a significant predictor of
complications and premature mortality (Muangchan
et al., 2012; Darby et al., 2016). Hence, daily
subcutaneous injections also were associated with
inflammation under panniculus carnosus in both
animal groups. Probably, the increase in porphyrin
fluorescence intensity in both groups was due to it.
Nevertheless, to prove our assumption a separate
study needs to be carried out providing with the
method development that will make it possible to
distinguish chronic hypoxia and inflammation of
different sites.
4 CONCLUSIONS
The use of optical technologies in the experiment
enabled to determine the increment in endogenous
fluorescence intensity of collagen and the decrease
in tissue oxygen intake in the fibrosis area. We also
registered the increase in endogenous fluorescence
intensity rate of the porphyrins as a potential chronic
hypoxia and inflammatory marker.
It is important that all optical methods used in
this study were non-invasive. Nevertheless we
managed to obtain quantitative and impersonal
information. Considering the fact that the animal
scleroderma model is relevant, the data obtained can
be reproduced in man.
The results of the experiment, of course,
demonstrate the necessity of a research continuation
in this direction, but already now it is possible to
predict great opportunities for optical technologies
in the diagnosis of skin fibrosis.
REFERENCES
Avouac, J., 2014. Mouse Model of Experimental Dermal
Fibrosis: The Bleomycin-Induced Dermal Fibrosis.
Arthritis Research: Methods and Protocols, 91-98.
Avouac, J., Elhai, M., Allanore, Y., 2013. Experimental
models of dermal fibrosis and systemic sclerosis. Joint
Bone Spine, 80 (1), 23-28.
Bataller, R., Brenner, D. A., 2005. Liver fibrosis. Journal
of clinical investigation,115 (2), 209-218.
Clements, P. J., Clements, P.J., Hurwitz, E.L., Wong, W.
K., Seibold, J.R, Mayes, M., White, B., Wigley, F.,
Weisman, M., Walter Barr, W., Larry Moreland, L.,
Medsger, Jr.Th.A., Steen, V.D., Martin, R.W., Collier,
D., Weinstein, A., Lally, E., Varga, J., Weiner, S.R.,
Andrews, B., Abeles, M., Furst, D.E., 2000. Skin
thickness score as a predictor and correlate of outcome
in systemic sclerosis: high‐dose versus low‐dose
penicillamine trial. Arthritis & Rheumatism, 43 (11),
2445-2454.
Driskell, R.R., Lichtenberger, B.M.,
Hoste, E.,
Kretzschmar, K., Simons, B.D., Charalambous, M,
Ferron, S.R., Herault, Y, Pavlovic, G, Ferguson-Smith,
A.C.,
and Watt F.M., 2013. Distinct fibroblast lineages
determine dermal architecture in skin development and
repair. Nature, 504 (7479), 277-281.
Darby, I. A., Hewitson, T. D, 2016. Hypoxia in tissue
repair and fibrosis. Cell and tissue research, 365 (3),
553-562.
Iredale, J. P., 2007. Models of liver fibrosis: exploring the
dynamic nature of inflammation and repair in a solid
organ. Journal of clinical investigation, 117 (3), 539-
548.
Franco, W., Gutierrez-Herrera, E., Kollias, N., Doukas,
A., 2016. Review of applications of fluorescence
excitation to spectroscopy dermatology. British
Journal of Dermatology, 174 (3), 499-504.
Gabrielli, A., Avvedimento, E. V., Krieg, T., 2009.
Scleroderma. New England Journal of Medicine, 360
(19), 1989-2003.
Hinz, B.,, Phan, S.H., Thannickal, V.J., Prunotto, M.,
Desmoulière, A., Varga, J., De Wever, O., Mareel, M.,
Gabbiani, G. 2012. Recent developments in
myofibroblast biology: paradigms for connective
tissue remodelling. The American journal of
pathology, 180.(4),.1340-1355.
Ho,Y.Y., Lagares, D., Tager, A.M., Kapoor, M., 2014.
Fibrosis - a lethal component of systemic sclerosis.
Nature Reviews Rheumatology, 10 (7), 390-402.
Jinnin M., 2010. Mechanisms of skin fibrosis in systemic
sclerosis. The Journal of dermatology, 37 (1), 11-25.
Kang,T., Abignano,
G., Lettieri,
G., Wakefield,
R.J.,
Emery, P., and Del Galdo, F., 2104. Skin imaging in
systemic sclerosis. European journal of rheumatology,
1 (3), 111-116.
Khanna, D., Denton, C. P., 2010. Evidence-based
management of rapidly progressing systemic sclerosis.
Best practice & research Clinical rheumatology, 24
(3), 387-400.
BIODEVICES 2018 - 11th International Conference on Biomedical Electronics and Devices
198

Lokmic, Z., Musyoka, J., Hewitson, T. D., & Darby, I. A.,
2012. 3 Hypoxia and Hypoxia Signaling in Tissue
Repair and Fibrosis. International review of cell and
molecular biology, 296, 139-185.
Maurer, B., Graf, N., Michel, B.A., Müller-Ladner, U.,
Czirják, L., Denton, C.P., Tyndall, A., Metzig, C.,
Lanius, V., Khanna, D., Distler, O., 2014. Prediction
of worsening of skin fibrosis in patients with diffuse
cutaneous systemic sclerosis using the EUSTAR
database. Annals of the rheumatic diseases, 205226.
Manresa, M. C., Godson, C., Taylor, C. T., 2014.
Hypoxia-sensitive pathways in inflammation-driven
fibrosis. American Journal of Physiology-Regulatory,
Integrative and Comparative Physiology, 307 (12),
1369-1380.
Monstrey, S., Hoeksema, H., Verbelen, J., Pirayesh, A.,
Blondeel, P., 2008. Assessment of burn depth and
burn wound healing potential. Burns, 34 (6), 761-769.
Muangchan, C., Harding, S., Khimdas, S., Bonner, A.,
Canadian Scleroderma Research Group, Baron, M.,
Pope, J., 2012. Association of C‐reactive protein with
high disease activity in systemic sclerosis: Results
from the Canadian Scleroderma Research Group.
Arthritis care & research, 9, 1405-1414.
Petritskaya, E.N., Kulikov, D.A., Rogatkin, D.A., Guseva,
I.A., and Kulikova, P.A., 2015. Use of fluorescence
spectroscopy for diagnosis of hypoxia and
inflammatory processes in tissue. Journal of Optical
Technology, 82 (12), 810-814.
Smirnova, O. D., Rogatkin, D. A., Litvinova, K. S., 2012.
Collagen as in vivo quantitative fluorescent
biomarkers of abnormal tissue changes. Journal of
Innovative Optical Health Sciences, 5 (02), 1250010.
Rockey, D. C., Bell, P. D., Hill, J. A., 2015. Fibrosis—a
common pathway to organ injury and failure. New
England Journal of Medicine, 372 (12), 1138-1149.
Rogatkin, D. A., Lapaeva, L. G., Petritskaya, E. N.,
Sidorov, V. V., and Shumskiy, V. I., 2009.
Multifunctional laser noninvasive spectroscopic
system for medical diagnostics and metrological
provisions for that. Clinical and Biomedical
Spectroscopy, 73681.
Rogatkin, D., Shumskiy, V., Tereshenko, S., Polyakov,
P., 2013. Laser-based non-invasive
spectrophotometry–An overview of possible medical
applications. Photonics & Lasers in Medicine, 2(3),
225-240.
Van Hal, T. W., Van Bon, L., Radstake, T. A, 2011.
System out of breath: how hypoxia possibly
contributes to the pathogenesis of systemic sclerosis.
International journal of rheumatology, 2011, 824972.
Wermuth, P. J., Jimenez, S. A., 2015. The significance of
macrophage polarization subtypes for animal models
of tissue fibrosis and human fibrotic diseases. Clinical
and translational medicine, 4 (1), 2.
Wynn, T. A., 2011. Integrating mechanisms of pulmonary
fibrosis. Journal of Experimental Medicine, 208 (7),
1339-1350.
Wynn, T. A., 2008. Cellular and molecular mechanisms of
fibrosis. The Journal of pathology, 214 (2),199-210.
Yamamoto, T., Katayama, I., 2011. Vascular changes in
bleomycin-induced scleroderma. International journal
of rheumatology, 2011, 270938.
Optical Technology for Fibrotic Skin Changes Objectification in Experimental Systemic Scleroderma
199
