
Quantitative Measurement of Bradykinesia in Parkinson's Disease
using Commercially Available Leap Motion
Yusuf Özgür Çakmak
1
, S. Can Ölçek
2
, Burak Özsoy
3
and Didem Gökçay
2
1
Department of Anatomy, University of Otago, Dunedin, New Zealand
2
Department of Health Informatics, Middle East Technical University, Graduate School of Informatics, Ankara, Turkey
3
Global Dynamic Systems (GDS) ARGE, Teknopark Istanbul, Istanbul, Turkey
Keywords: Parkinsons, Bradykinesia, Updrs, Quantitative, Leap Motion, Pinching.
Abstract: Parkinson’s Disease (PD) is a neurodegenerative disease caused by the depletion of dopamine in the brain.
Tremor, bradykinesia, rigidity and postural stability are the four major symptoms. Like other symptoms,
bradykinesia causing unnatural stillness/slowness in motions affects the daily life of the patients. The levels
of these symptoms are clinically assessed by a scoring system based on Unified Parkinson's Disease Rating
Scale (UPDRS). However, UPDRS relies on the visual observations of physicians rather than a test based
on quantitative measurements. This makes it not only difficulty to repeat but also subjective. Because of
these two major disadvantages, researchers build custom devices for their studies. But this leads to the
reliability issues and non-standard measurements. Thus, 24 PD patients were bilaterally UPDRS III (motor
subsection) scored and recorded for finger motion (pinching) by using commercially available off-the-shelf
(COTS) product called Leap Motion. The various features extracted from recordings and UPDRS III scores
were analyzed for correlation. After the analysis, a linear model was created to estimate UPDRS III score.
The study revealed that Leap Motion, a COTS device, can be used to estimate bradykinesia of a patient with
PD.
1 INTRODUCTION
Bradykinesia which results in unnatural
stillness/slowness in the motions is one of the early
symptoms of Parkinson's Disease (PD). Together
with tremor, rigidity, and postural instability, they
are named as four cardinal symptoms of the disease
(Calne et al., 1992). The main cause of bradykinesia
is the dopamine deficiency in basal ganglia from
which the inhibitory signals are sent to the motor
systems to prevent involuntary actions. Under
normal circumstances when the dopamine is present,
basal ganglia promotes those motor actions so that
the body can act swiftly (Blandini et al., 2000).
Because of further depletion of dopamine in later
stages, bradykinesia follows the progression of the
disease and it gets worse.
The level of disease and its symptoms are
evaluated by Unified Parkinson's Disease Rating
Scale (UPDRS). UPDRS scoring, which is based on
the observations of the physician conducting it, is
the main clinical approach to diagnose and assess
the progression of the disease. Even though UPDRS
III (motor subsection) covers almost all the aspects
of the motor symptoms (Fahn et al., 1987) it depends
on the subjective scoring of the physicians. In
addition to this inconsistency, the discreet rating
scale cannot detect the subtle changes in the
symptoms such as bradykinesia. Therefore, UPDRS
solely is not adequate for research or treatment of
PD.
Various researchers (Dunnewold et al., 1997;
Salarian et al., 2007; Kandori et al., 2004; Ghassemi
et al., 2006; Sande de Souza et al., 2011; Marsili et
al., 2014; Daneault et al., 2013) have tried many
different assessment techniques to overcome the
inadequacy of UPDRS for detecting bradykinesia.
All these techniques are mostly focused on rapid
alternating movements (RAM) or finger
tapping/pinching. For example, Dunnewold et al.
(1997) used tap rate (TR) and movement time (MT)
to assess slowness in the motion. Similarly,
Ghassemi et al. (2006) used another RAM which is
pronation-supination action to measure bradykinesia.
However, in Ghassemi et al.’s work, the pronation-
supination action did not show a significant
Çakmak, Y., Ölçek, S., Özsoy, B. and Gökçay, D.
Quantitative Measurement of Bradykinesia in Parkinson’s Disease using Commercially Available Leap Motion.
DOI: 10.5220/0006655402270232
In Proceedings of the 11th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2018) - Volume 4: BIOSIGNALS, pages 227-232
ISBN: 978-989-758-279-0
Copyright © 2018 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
227

correlation with the bradykinesia level unlike the
tapping and alternating hand movements used in
other studies. Nonetheless, Daneault et al. (2013)
clarified those odd findings by showing that the
maximal and mean velocity of pronation-supination
cycles has significant correlation rather than the
cycle duration. Even though all the studies agree that
RAM based tasks can be used in assessing
bradykinesia level, the measurement techniques are
relying on wide variety of devices such as
accelerometers (Dunnewold et al., 1997),
gyroscopes (Salarian et al., 2007), magnetic devices
(Kandori et al., 2004; Ghassemi et al., 2006), and
EMG sensors (Sande de Souza et al., 2011). The
common problem of all these devices is that they are
depending on custom designs or setups. In other
words, they are not commercially available off-the-
shelf (COTS) products.
The objective of this study is to develop a new
method to measure bradykinesia in PD patients by
using COTS product called Leap Motion. Thus, the
efficiency of Leap Motion is studied by recording
various motor tasks performed by PD patients. The
recorded data is analyzed for its various features
against the UPDRS scores. The aim is to be able to
utilize this easily available and relatively cheap
device for daily tracking of patients and their
treatments. The study is approved by the local Ethics
Committees of Koç University Hospital, İstanbul,
Turkey and all participants gave informed consent
prior to the study.
2 METHODS
2.1 Measurement Device
Leap Motion (Leap Motion, Inc., San Francisco,
USA) is a motion controller device to capture hand
gestures by using pair of cameras and infrared
lighting. It is a fairly compact device and very
powerful to capture obvious hand motions like
pinching and pronation/supination. Figure 1 shows
the device interior and its compact design.
Figure 1: The representation of interior design of Leap
Motion taken from its product page.
Weichert et. al. (2013) analyzed the accuracy of
leap motion controller and found that it can achieve
0.7 mm overall average accuracy in all 3 axes. This
result is comparable to the average human hand
accuracy, 0.4 mm. Besides the accuracy, the
controller is able to sample the hand motions around
100 Hz.
2.2 Recorded Motor Tasks and
Features
Pinching and Pronation-Supination are the two
motor tasks given to the subjects. In this study, we
will report preliminary results from the pinching task
only. Other data will be reported separately. With
the software developed on top of Leap Motion SDK,
the positions and rotations of the finger joints and
wrist are recorded during these tasks. After the
recording session, the raw data is processed and
several features are extracted. For the pinching, the
local minima and maxima of the distances between
thumb and index finger are marked. Afterwards, the
time difference between the consecutive minimum
and maximum is calculated.
By using the time difference and distance
obtained from the raw data processing, the speed,
acceleration, and frequency of a motion are
calculated. In previous studies, it was shown that
those three measures can be used to assess
bradykinesia. (Dunnewold et al., 1997; Daneault et
al., 2013)
2.3 Subjects and Experiment Protocol
24 patients (7 female, 17 male, mean age ± SD =
57.08 ± 8.91) who were diagnosed by neurologist
for PD participated in the experiment. All patients
were under dopaminergic replacement treatment and
their disease duration was 8.04 ± 3.88 years. 20
patients were right-handed whereas 4 patients were
left-handed. They came to the hospital in 12-hour
OFF state (without medication) and two independent
neurologists immediately evaluated UPDRS III
(motor section) bilaterally. The average of those two
scorings was considered as the final bilateral
UPDRS scores (
). The patients
were not specifically marked as tremor or
bradykinesia dominant.
The patients visited hospital multiple times for
another ongoing study for the data acquisition. There
was at least one week difference between visits. 9
patients came to hospital twice and 15 remaining
patients were recorded three times. In every case, the
BIOSIGNALS 2018 - 11th International Conference on Bio-inspired Systems and Signal Processing
228

patients were seated against a laptop computer to
which the leap motion controller is connected. The
controller was laid on the table. To familiarize the
patients with the device and to test the setup, they
were asked to put their hand above the controller and
move their fingers as shown in Figure 2. It was
visually verified that the controller was capturing the
gestures.
Figure 2: Basic recording setup with laptop and leap
controller.
After the initial UPDRS scoring and familiariza-
tion was completed, the participant started to
experiment. During the study, the motor tasks given
to the patients were recorded in 3 successive
sessions for both hands. Namely, one patient has
total 12 recordings (6 pinching, 6 wrist motion) per
hospital visit. At the end of the data acquisition
phase, total 378 recordings were taken for pinching.
The important part of the study is that before each
session, bilateral UPDRS III scoring was evaluated
by the same neurologists. The reason for the
repeated scoring is to capture the subtle changes in
the symptoms between the visits and different
sessions. Each motion task was recorded at least 10
seconds for both hands one after another.
2.4 Analysis and Statistics
Regardless of the session and action hand, the
feature extraction was applied onto all recordings.
Because of the fixation problems observed in the
data (Figure 3), the first several extracted values of
each feature (time difference, distance, and angles)
were removed. With remaining features, the mean
and standard deviation of speed, acceleration, and
frequency were calculated. By comparing the mean
and standard deviation of each metric, it was decided
if the patient could perform the task correctly or not.
Table 1 lists several exemplary values discarded
because of having large deviations. In other words,
the examples in the table have SD values which are
almost comparable to the corresponding mean
values.
Figure 3: Change of the distance between thumb and index
finger during pinching for 3 different patients. The
fixation problem can be seen at the start (before 2 seconds)
of signal where the pattern is distorted.
Table 1: Discarded speed values because of large SD.
Mean Value (mm/s)
SD (mm/s)
216.80
129.36
689.53
543.78
Since the bilateral UPDRS scores were
independently taken before each session, the values
calculated for both hands were pooled together as
Marsili et al. (2014) did. Similarly, the recordings of
all the visits and their three distinct sessions were
also combined. This data pooling process was done
separately for each motor task. After obtaining the
Quantitative Measurement of Bradykinesia in Parkinson’s Disease using Commercially Available Leap Motion
229

two big sets of recordings, the correct metric was
selected for pinching and pronation-supination,
respectively. Thus, Pearson’s correlation was
applied between UPDRS scores and three metrics
derived from extracted features.
Later, by using all the metrics of both motor
tasks, a linear regression model as in Equation 1 was
derived to improve the link between UPDRS III and
the data gathered from the controller. The
correctness of the model was evaluated by the root-
mean-square error defined by Equation 2.
(1)
(2)
3 RESULTS
Some patients couldn’t complete the tasks given to
them. There were 9 such sessions that were excluded
from the study. Unrelated to the data content, the
data belonging to one patient were discarded
because of invalid UPDRS scoring. The features of
43 pinching recordings couldn’t be extracted
because of invalid or missing data. As a result, these
43 data were also removed from the data pool.
The investigation of mean and standard deviation
of metrics calculated for remaining sessions revealed
that almost half of the data for each metric have
large deviations (
). Since it is not possible
to include these inconsistent values, the correlation
study was completed by discarding them.
Firstly, the pinching task was analyzed and it
was found that there were very low correlations
(
) between the pinching metrics and their
respective contra-lateral UPDRS III scores.
However, when the analysis was conducted against
the ipsi-lateral scores, a moderate correlation was
obtained (
). UPDRS III motor section
contains many items focusing on a specific
symptom. Thus, the correlation study was repeated
against the bradykinesia subset of UPDRS III
because the pinching performance should be mostly
affected by bradykinesia. As expected, the results
(
) got better for all three metrics. In the
end, the speed is the best metric for the pinching.
Even though the speed was selected as the best
metric for pinching, the values were fitted to create
linear model from all metrics as in Equation 3 (
) to
estimate UPDRS III score.
(3)
(4)
The correlation between pinching and
bradykinesia was significant so should be the linear
model when the features of pinching is selected as
sole predictors. The important point is that this
model had small root-mean-square error (
) for estimating total UPDRS III score. To
better visualize the error, it is normalized (
) by the max value of UPDRS III as in
Equation 4.
Because of stronger correlation with
bradykinesia subset in pinching, the linear model
was also created for UPDRS III bradykinesia score.
As expected, the error of this model was similarly
small (
). Even though the
normalized value was slightly bigger than the error
in the total score case, it was not significantly
different.
Instead of using whole data to create the model,
the training procedure was repeated by using
randomly selected 75% of the data. After training,
the remaining 25% of the data was used for testing
the model. This training-testing procedure was
repeated 100 times for the different randomly
selected training set. After 100 repetitions, the
average RMSE values were calculated. The results
of trained model were similar to the previous
approach for both total UPDRS III (
and bradykinesia subset (
cases. The important finding was that
error of estimations was (
and (
respectively.
4 DISCUSSIONS
In this study, we showed that a COTS device can be
used in simple setup to assess the bradykinesia level
of the patient with PD. Furthermore, it is important
that the assessment was done by using a quantitative
metric acquired from the device. By comparing the
measurements with the UPDRS III scores which are
based on the subjective observations of physicians, it
was seen that this method can be used as a fast and
BIOSIGNALS 2018 - 11th International Conference on Bio-inspired Systems and Signal Processing
230
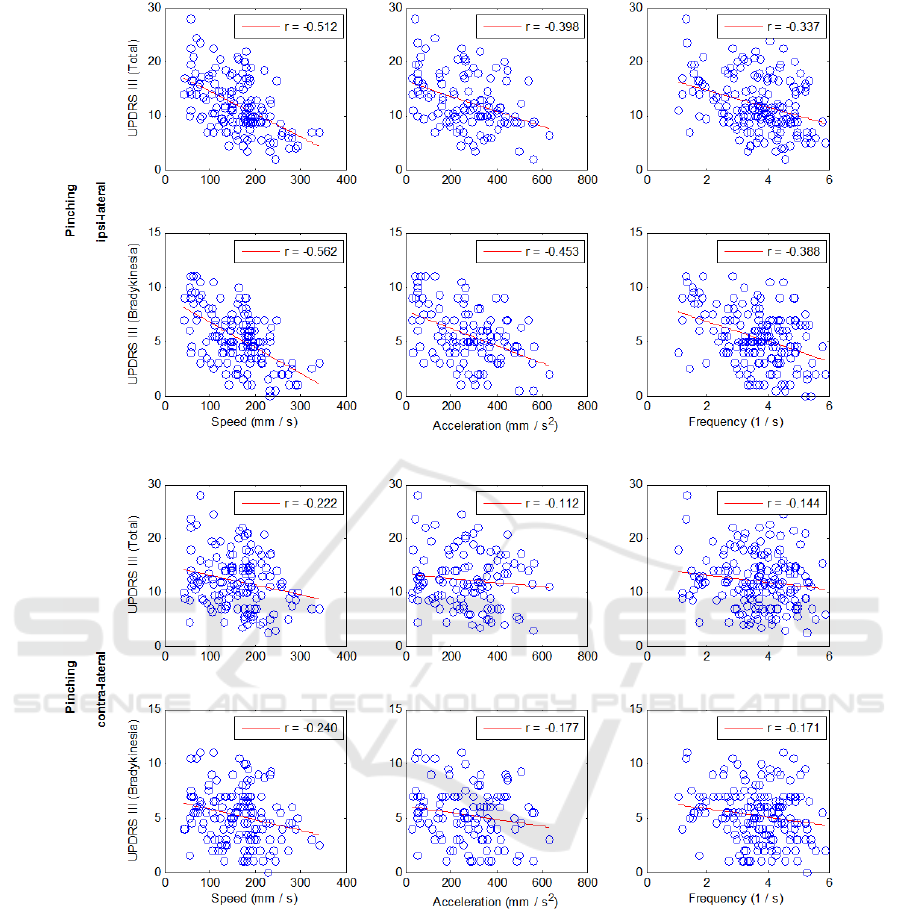
Figure 4: Distribution of UPDRS III total score and bradykinesia subset against the three metrics (speed, acceleration,
frequency) extracted from the pinching recordings. The metrics are the combination of data from all the session for both
hands. The upper two rows show the ipsi-lateral results while the bottom two rows belong to contra-lateral results. As
expected, the speed showed the highest correlation ( and the correlation (
increases by using bradykinesia subset. Furthermore, the contra-lateral analysis revealed that there was no
correlation between the total ( ) and the metrics. Even using bradykinesia subset (
scores did not improve it.
reliable alternative. The main advantage of this
technique is that it helps the physician by keeping
the process completely objective, thus, they can
better decide on treatment regime. Nevertheless, the
number of invalid data suggested that the patients
need further familiarization with the task and device.
This can be overcome by extending the recording
time and the familiarization time. The exclusion of
data could be done by using z-scores of the metrics
which might give further information why the
patients couldn’t complete the given task.
UPDRS is a subjective scoring system, although
it is widely used in the clinic. Due to its subjectivity,
having mild to moderate correlations of UPDRS
Quantitative Measurement of Bradykinesia in Parkinson’s Disease using Commercially Available Leap Motion
231

with an actual physical measure is not surprising.
Despite this fact, UPDRS III was chosen for the
validation because it is the clinical golden standard
for diagnosis and prognosis. The correlation study
revealed that the fine movements like pinching
expresses bradykinesia well. Further testing of linear
model showed that this method is less error-prone
than the UPDRS. If a physician makes 1 scale-unit
error for each item, the error becomes
which is a value much larger than our proposed
model’s error.
5 CONCLUSION
The study proved that a commercially available
cheap Leap Motion device can be used to measure
bradykinesia level from simple motor tasks. In
comparison to UPDRS scoring relying on the
physicians’ observations, it provides repeatable and
quantitative measurements. These two major
advantages of technique make it suitable for research
purposes where the detection of subtle changes in
symptoms is required. The possibility of using a
COTS device can be an invaluable asset for other
researchers. With further investigations such as
comparison with the results of another clinical
physiologic sensor, Leap Motion can be converted to
the household self-assessment device. Unfortuna-
tely, in our study, the data exclusion rate was high,
which calls for attention to investigate further the
applicability of this procedure in the clinic.
REFERENCES
Blandini, F., Nappi, G., Tassorelli, C. and Martignoni, E.
(2000). Functional changes of the basal ganglia
circuitry in Parkinson's disease. Progress in
Neurobiology, 62(1), pp.63-88.
Calne, D., Snow, B. and Lee, C. (1992). Criteria for
diagnosing Parkinson's disease. Annals of Neurology,
32(S1), pp.S125-S127.
Daneault, J., Carignan, B., Sadikot, A. and Duval, C.
(2013). Are quantitative and clinical measures of
bradykinesia related in advanced Parkinson's disease?.
Journal of Neuroscience Methods, 219(2), pp.220-223.
Dunnewold, R., Jacobi, C. and van Hilten, J. (1997).
Quantitative assessment of bradykinesia in patients
with Parkinson's disease. Journal of Neuroscience
Methods, 74(1), pp.107-112.
Fahn, S., Marsden, C., Goldstein, M. and Calne, D.
(1987). Recent developments in Parkinson's disease.
Volume 2. Florham Park: Macmillan Healthcare
Information, pp.153–163.
Ghassemi, M., Lemieux, S., Jog, M., Edwards, R. and
Duval, C. (2006). Bradykinesia in patients with
Parkinson's disease having levodopa-induced
dyskinesias. Brain Research Bulletin, 69(5), pp. 512-
518.
Kandori, A., Yokoe, M., Sakoda, S., Abe, K., Miyashita,
T., Oe, H., Naritomi, H., Ogata, K. and Tsukada, K.
(2004). Quantitative magnetic detection of finger
movements in patients with Parkinson’s disease.
Neuroscience Research, 49(2), pp.253-260.
Marsili, L., Agostino, R., Bologna, M., Belvisi, D., Palma,
A., Fabbrini, G. and Berardelli, A. (2014).
Bradykinesia of posed smiling and voluntary
movement of the lower face in Parkinson's disease.
Parkinsonism & Related Disorders, 20(4), pp. 370-
375.
Salarian, A., Russmann, H., Wider, C., Burkhard, P.,
Vingerhoets, F. and Aminian, K. (2007).
Quantification of Tremor and Bradykinesia in
Parkinson's Disease Using a Novel Ambulatory
Monitoring System. IEEE Transactions on Biomedical
Engineering, 54(2), pp.313-322.
Sande de Souza, L., Dionísio, V. and Almeida, G. (2011).
Multi-joint movements with reversal in Parkinson’s
disease: Kinematics and electromyography. Journal of
Electromyography and Kinesiology, 21(2), pp.376-
383.
Weichert, F., Bachmann, D., Rudak, B. and Fisseler, D.
(2013). Analysis of the Accuracy and Robustness of
the Leap Motion Controller. Sensors, 13(5), pp.6380-
6393.
BIOSIGNALS 2018 - 11th International Conference on Bio-inspired Systems and Signal Processing
232
