
Application of Zero-Valent Iron Nanoparticles for Diclofenac
Removal
Nianqing Zhou
1
,Wen Liang
1,*
,Chaomeng Dai
1,2
and Yanping Duan
3
1
College of Civil Engineering, Tongji University, Shanghai 200092, China;
2
State Key Laboratory of Petroleum and Petrochemical Pollution Control and Treatment, Shanghai 200092, China;
3
Institute of Urban Study, Shanghai Normal University, Shanghai 200234, China.
Email:yclwvean@163.com
Keywords
: nZVI, diclofenac, oxidation, Fenton-like
Abstract:
Application of zero-valent iron nanoparticles (nZVI) for DCF removal and its mechanism were discussed.
With the solids concentration of 0.5 g/L nZVI, more than 30% of DCF could be removed rapidly in 5 min.
The pH value and dissolved oxygen (DO) were the important factors of DCF removal by nZVI. Under
acidic condition, the DCF removal efficiency was relatively high, during to the oxidation of Fenton-like
system. Under neutral and alkaline conditions, the DCF removal efficiency was low, because of the low
capacity adsorption of the FeOOH-shell. This study has provided the basis for DCF removal by nZVI-
Fenton-like system.
1 INTRODUCTION
Trace level of pharmaceuticals have been reported in
natural environments because of the widespread use
(Alvarino et al., 2015;
Alvarino et al., 2014; Liu et
al., 2014). Diclofenac (DCF), a non-steroidal anti-
inflammatory drug, is widely applied as a pain killer,
which has been one of the most frequently detected
pharmaceuticals in surface water and groundwater,
due to its’ poor treatability in municipal sewage
treatment plants (STPs) (Castiglioni et al., 2006;
Vieno and Sillanpää, 2014). Studies have shown that
DCF residues and their metabolites in water bodies
can produce biotoxic effects on different living
organisms in water environment, which can lead to
microbial resistance and cross resistance. DCF in
effluent from STPs may also cause downstream
aquatic and terrestrial ecology. The toxic effects of
the system pose a great threat to the environment
and human health (Stülten et al., 2008;
Dai et al.,
2009; Boxall et al., 2003). Therefore, DCF removal
technologies need to be further discussed.
Nanoscale zero-valent iron (nZVI) has been
investigated as a green in-situ tool for the
degradation of both organic and inorganic
contaminants for more than 10 years (Liang et al.,
2014; Han et al., 2016; Hwang et al., 2015; Sheng et
al., 2016; Li et al., 2015) The successful application
of nZVI in organic contaminants degradation was
explored and reported by many researchers (Xia et
al., 2014; Machado et al., 2013; Noradoun et al.,
2003).
In this study, the DCF removal mechanism by
nZVI was investigated based on the operation
conditions, including nZVI solids loading, pH value
and dissolved oxygen (DO).
2 MATERIALS AND METHODS
2.1 Chemicals and Materials
Diclofenac sodium (C
14
H
10
Cl
2
NNaO
2
, 99%), ferric
chloride anhydrous (FeCl
3
, 99%), sodium
borohydride (NaBH
4
, 98%), and sodium hydroxide
(NaOH, 99%) were obtained from Aladin.
Hydrochloric acid (HCl, 37%) was purchased from
Sinopharm Chemical Reagent Shanghai Co., Ltd.
Methanol (HPLC grade), acetonitrile (HPLC grade),
and acetic acid (HPLC grade) were obtained from
Sigma-Aldrich. All chemicals were used without
further purification.
Zhou, N., Liang, W., Dai, C. and Duan, Y.
Application of Zero-Valent Iron Nanoparticles for Diclofenac Removal.
In Proceedings of the International Workshop on Environment and Geoscience (IWEG 2018), pages 87-91
ISBN: 978-989-758-342-1
Copyright © 2018 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
87

Deionized water was prepared with a Milli-Q
water purification system (Millipore, Bedford, MA,
USA). Microporous membranes (0.22 μm×50 mm)
were obtained from CNW (Germany).
2.2 Synthesis of NZVI
The nZVI was synthesized according to the method
of liquid-phase reduction of ferric trichloride by
sodium borohydride (Sun et al., 2006). The sodium
borohydride (NaBH4, 0.5 M) and ferric chloride
anhydrous (FeCl
3
, 0.1 M) with the volume ratio of
1:1 were vigorously reacted. Then the generated jet-
black nZVI particles were collected through vacuum
filtration and respectively washed with deionized
water for three times. Finally, fresh nZVI particles
were stored in deionized water by blowing nitrogen
at 4 °C.
2.3 Characterization of NZVI
The high-resolution transmission electron
microscopy (TEM) observation was performed
using a JEOL JEM 2011 HR-TEM operated at 200
kV with an INCA EDS system.
2.4 Batch Experiments
A 1.0 g/L stock solution of DCF was prepared with
deionized water. Uptake reactions were initiated by
the addition of nZVI particles into 150 mL DCF
solution without pH adjusted. The nZVI loading
concentration in the solution was 0.1, 0.2, 0.3, 0.4,
0.5, 0.8, 1.0, and 2.0 g/L, respectively, at a DCF
concentration of 1 mg/L. After mixing, the reactors
were continuously shaken for 2 hours on an orbital
shaker. The optimum loading of nZVI was obtained
by comparing the results of the above experiments.
All the experiments were performed in triplicate.
To investigate the effect of solution pH on DCF
removal by nZVI, the initial solution pH was
adjusted from 3 to 9 with the initial DCF
concentration at 1 mg/L by small amounts of HCl or
NaOH solution. Then water samples with different
pH values were applied to 0.5 g/L nZVI. All the
experiments were performed in triplicate.
The effect of oxygen on DCF removal by nZVI
was investigated under the DO-limiting with the
optimum nZVI loading and pH value. The oxygen-
limiting condition was established by blowing
nitrogen over the solution with nitrogen evaporator
(N-Evap-111, Organomation Associates, Inc.).
Nitrogen blowing time was kept at least 15 minutes
to ensure DO less than 0.5 mg/L. The concentration
of DO was monitored by dissolved oxygen meter
(HQ-30D, Hach Co.). The initial solution pH value
was controlled at 3 and 5. Reaction time was 5, 10,
15, 30, 60, 90 and 120 min, respectively. All the
experiments were performed in triplicate.
All solution samples were filtered with 0.22 μm
membrane before analysis. The concentrations of
DCF in the sample were determined by high
performance liquid chromatography (HPLC, Agilent
1260) equipped with an EC-C18 packed column
(Agilent). Initial mobile phase of the analysis was a
mixture of 30% deionized water (containing 0.1%
CH
3
COOH) and 70% acetonitrile. Final mobile
phase ratio was 75%: 25%, within 5 min. The
samples were measured at a rate of 1.0 ml/min at a
wave length of 275 nm. After measurement,
methanol was used to clean the EC-C18 column. All
the experiments were performed in triplicate.
2.5 Statistical Analyses
One-way ANOVA was performed to assess the
experimental data. Statistical significance was
evaluated at p<0.05 level. The SPSS software (Ver
20.0) was applied for all statistical analyses.
3 RESULTS AND DISCUSSION
3.1 Characterization Of NZVI
Fresh nZVI particles were analyzed by transmission
electron microscopy (TEM). The iron particles were
typically less than 100 nm in diameter. Figure 1
showed the smooth sphere surrounded the core
structure, indicated that oxidation happened on the
surface.
Figure 1: The TEM analysis of fresh nZVI particles.
IWEG 2018 - International Workshop on Environment and Geoscience
88
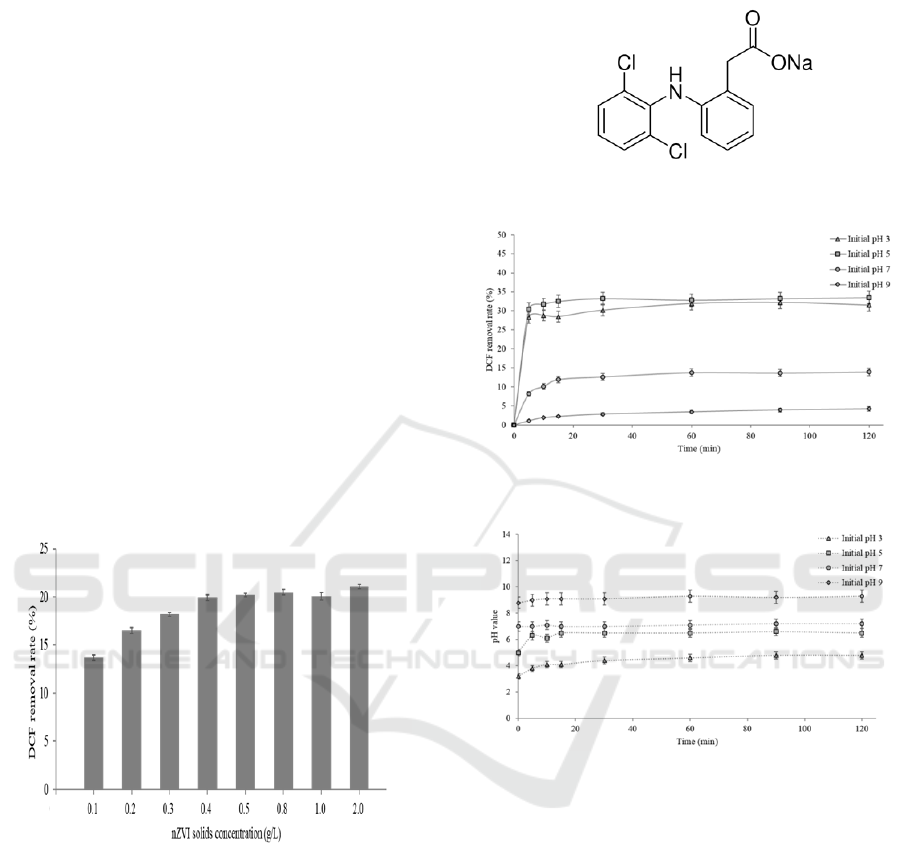
3.2 DCF Removal By NZVI
Different solids concentration was prepared for the
uptake experiments at the ranging from 0.1 to 2 g/L
nZVI at a DCF concentration of 1 mg/L,
respectively for 2 h. The solution kept the initial pH
value of 6.7 without adjusting. As shown in Figure 2,
with the increase of nZVI loading, the removal
efficiency of DCF was increased. When nZVI
loading was higher than 0.4 g/L, the removal
efficiency was over 20%. Thus, we defined 0.5 g/L
loading as the optimum solids concentration for 1
mg/L DCF treatment. And the loading was adopted
in the subsequent experiments.
The nZVI particles have been widely applied in
environment remediation due to its complex
contaminant removal pathways, including
adsorption, complexation, (co)precipitation and
surface-mediated chemical reduction (Miehr et al.,
2004). As its structure and characteristic, surface-
mediated chemical reduction is likely not the reason
of DCF removal by nZVI.
Figure 2: Effect of nZVI solids concentration on DCF
removal.
3.3 Effect of Solution PH
The pH is an important factor for DCF removal by
nZVI. DCF is a weak acid with a pKa of 4, and as
shown in Figure 3, DCF has the carboxylic group
and the NH group, which can be act either as proton
donor or proton acceptor, so that it possesses a
Lewis acid-base character (Žilnik et al., 2007).
When the pH of the solution is less than 4, DCF
carries positive charge, and negative charge when
the pH of the solution is greater than 4.
Figure 3: Molecular structure of DCF.
Figure 4: Effect of pH value on DCF removal.
Figure 5: Variation of pH value during DCF removal.
The freshly prepared 0.5 g/L nZVI particles were
injected into the sample with 1 mg/L DCF
concentration for 2 h. Figure 4 has shown the uptake
results at various pH conditions. The removal
efficiency in acidic condition was much higher than
neutral and alkaline. In acidic condition, the removal
efficiency was rapidly reached 30% within 5 min.
While under neutral and alkaline conditions, the
reaction was carried out relatively slow. In condition
of initial pH 5, the best removal result was obtained.
The removal trends were similar in condition of
initial pH 5 and pH 3. While in condition of initial
pH 9, the uptake rate was less than 5% after 2 hours’
reaction. The variation of pH in different initial
values was shown in Figure 5. A small increase in
pH value was observed under neutral and alkaline
Application of Zero-Valent Iron Nanoparticles for Diclofenac Removal
89

conditions. The pH increased relatively under acidic
condition, but still below 7.
The nZVI particles were corroded by acid and
oxygen. During the corrosion of iron by acid,
ferrous iron and ferric iron solution could be
generated based on Equations (1), (2) and (3) (Sun
et al., 2006;
Kishimoto et al., 2011). The present of
H
+
inhibited the formation of iron (oxy)hydroxide,
resulting in the low contribution of adsorption. In
neutral and alkaline conditions, the FeOOH-shell
could form based on Equations (4), (5) and (6) (Sun
et al., 2006;
Kishimoto et al., 2011; Hœrléet al.,
2004). According to the equations, the nZVI
particles carry positive charge in condition of acid,
and negative charge in condition of alkali.
2
→2
↑ (1)
2
4
→2
2
(2)
4
4
→2
2
(3)
2
2
→2
(4)
4
2
→4
(5)
4
3
2
→4
(6)
There could be three main processes for the DCF
removal by nZVI: (1) pH in 7-9, both DCF and
nZVI carried negative charge, the physical
adsorption of FeOOH-shell was the leading reaction;
(2) pH in 4-7, DCF carried negative charge, while
nZVI carried positive charge, the chemical
adsorption might be one of the leading roles; (3) pH
in 3-4, both DCF and nZVI carried positive charge,
and adsorption could not be the leading roles in acid
condition, but in this process high DCF removal
efficiency was obtained, so oxidation of Fenton-like
system was the leading role, in which the corrosion
of iron by acid caused hydrogen peroxide to form, as
shown in Equation (7) and (8)
(Joo et al., 2004).
2
→2
(7)
→
∙
(8)
3.4 Effect of Dissolved Oxygen
For further study the DCF removal mechanism by
nZVI particles, the effect of dissolved oxygen (DO)
was examined.
The freshly prepared 0.5 g/L nZVI particles were
injected into the solution with 1 mg/L DCF
concentration for 2 h. The DO in system was
reduced to less than 0.5 mg/L by blowing nitrogen.
As shown in Figure 6, under DO-limiting condition,
both in pH 3 and in pH 5, the removal extent of DCF
was obviously reduced. The low efficiency of DCF
removal in pH 5 indicated that chemical adsorption
was not the leading role in pH 4-7.
Figure 6: Effect of DO on DCF removal.
3.5 Removal Mechanism
The pH and DO were the main limitation factors. In
different pH range, DCF showed different surface
charges, and nZVI showed different surface
structures. In Fenton-like system, DO was the main
donor of hydroxyl radical, and H
+
could corrode iron
particles to supply ferrous iron and ferric iron
solution. Figure 7 showed the main DCF removal
mechanisms by nZVI: (1) in pH 3-7, oxidation of
Fenton-like system was the leading role; (2) in pH 7-
9, physical adsorption of the FeOOH-shell was the
leading role, and less than 5% removal efficiency
showed that the capacity of nZVI adsorbed on DCF
was very low.
Figure 7: Schematic diagram of DCF removal mechanism
by nZVI.
IWEG 2018 - International Workshop on Environment and Geoscience
90

4 CONCLUSIONS
This study demonstrated that nZVI particles had a
removal effect on DCF. In pH 5, with the solids
concentration of 0.5 g/L nZVI, more than 30% of
DCF could be removed rapidly in 5 min. The pH
and DO were the main limitation factors. Under
acidic condition, the DCF removal efficiency was
relatively high, during to the oxidation of Fenton-
like system. Under neutral and alkaline conditions,
the DCF removal efficiency was low, because of the
low capacity adsorption of the FeOOH-shell. This
study has provided the basis for DCF removal by
nZVI-Fenton-like system. The follow-up study can
optimize the reaction conditions and enhance the
Fenton-like reaction to improve the removal
efficiency of DCF.
REFERENCES
Alvarino T, Suarez S, Katsou E, et al. 2015 Removal of
PPCPs from the sludge supernatant in a one stage
nitritation/anammox process Water Research 68 701
Alvarino T, Suarez S, Lema JM, et al. 2014
Understanding the removal mechanisms of PPCPs and
the influence of main technological parameters in
anaerobic UASB and aerobic CAS reactors Journal of
Hazardous Materials 278 506
Boxall ABA, Kolpin DW, Hallingsørensen B, et al. 2003
Peer Reviewed: Are Veterinary Medicines Causing
Environmental Risks? Environmental Science &
Technology 37 286A
Castiglioni S, Bagnati R, Fanelli R, et al., 2006 Removal
of pharmaceuticals in sewage treatment plants in Italy
Environ.sci.technol 40 357
Dai C, Zhou X, Zhang Y, et al. 2009 Research
advancements in potential risk of PPCPs of
environmental media Environmental Pollution &
Control
Han Y, Liu C, Horita J, et al. 2016 Trichloroethene
hydrodechlorination by Pd-Fe bimetallic nanoparticles:
Solute-induced catalyst deactivation analyzed by
carbon isotope fractionation Applied Catalysis B
Environmental 188 77
Hwang Y, Mines PD, Jakobsen MH, et al. 2015 Simple
colorimetric assay for dehalogenation reactivity of
nanoscale zero-valent iron using 4-chlorophenol
Applied Catalysis B Environmental 166-167 18
Joo SH, And AJF and Waite TD 2004 Oxidative
Degradation of the Carbothioate Herbicide, Molinate,
Using Nanoscale Zero-Valent Iron Environmental
Science & Technology 38 2242
Li Y, Cheng W, Sheng G, et al. 2015 Synergetic effect of
a pillared bentonite support on SE(VI) removal by
nanoscale zero valent iron Applied Catalysis B
Environmental 174-175 329
Liang W, Dai C, Zhou X, et al. 2014 Application of zero-
valent iron nanoparticles for the removal of aqueous
zinc ions under various experimental conditions Plos
One 9 e85686
Liu F, Zhao J, Wang S, et al. 2014 Effects of Solution
Chemistry on Adsorption of Selected Pharmaceuticals
and Personal Care Products (PPCPs) by Graphenes
and Carbon Nanotubes Environmental Science &
Technology 48 13197
Machado S, Stawiński W, Slonina P, et al. 2013
Application of green zero-valent iron nanoparticles to
the remediation of soils contaminated with ibuprofen
Science of the Total Environment 461-462 323
Miehr R, Tratnyek PG, Bandstra JZ, et al. 2004 Diversity
of contaminant reduction reactions by zerovalent iron:
role of the reductate Environmental Science &
Technology
38 139
Noradoun C, Mark D. Engelmann, Mclaughlin M, et al.
2003 Destruction of Chlorinated Phenols by Dioxygen
Activation under Aqueous Room Temperature and
Pressure Conditions Industrial & Engineering
Chemistry Research 42 5024
Sheng G, Yang P, Tang Y, et al. 2016 New Insights into
the Primary Roles of Diatomite in the Enhanced
Sequestration of UO22+ by Zerovalent Iron
Nanoparticles: An Advanced Approach Utilizing XPS
and EXAFS Applied Catalysis B Environmental 193
189
Stülten D, Zühlke S, Lamshöft M, et al. 2008 Occurrence
of diclofenac and selected metabolites in sewage
effluents Science of the Total Environment 405 310
Sun YP, Li XQ, Cao J, et al. 2006 Characterization of
zero-valent iron nanoparticles Advances in Colloid &
Interface Science 120 47
Vieno N, Sillanpää M, 2014 Fate of diclofenac in
municipal wastewater treatment plant - a review
Environment International 69 28
Xia S, Gu Z, Zhang Z, et al. 2014 Removal of
chloramphenicol from aqueous solution by nanoscale
zero-valent iron particles Chemical Engineering
Journal 257 98
Hœrlé S, Mazaudier F, Dillmann P, et al. 2004 Advances
in understanding atmospheric corrosion of iron. II.
Mechanistic modelling of wet–dry cycles Corrosion
Science 46 1431
Kishimoto N, Iwano S and Narazaki Y 2011 Mechanistic
Consideration of Zinc Ion Removal by Zero-Valent
Iron Water Air & Soil Pollution 221 183
Žilnik LF, Jazbinšek A, Hvala A, et al. 2007 Solubility of
sodium diclofenac in different solvents Fluid Phase
Equilibria 261 140
Application of Zero-Valent Iron Nanoparticles for Diclofenac Removal
91
