
High-temperature Creep Behavior of Al
x
CrMnFeCoNi High-
entropy Alloys
C M Cao, J Xu, Y X Hao, W Tong and L M Peng
*
CAS Key Laboratory of Mechanical Behavior and Design of Materials, Department
of Modern Mechanics, School of Engineering Science, University of Science and
Technology of China, Hefei, Anhui 230027, PR China
Corresponding author and email: L M Peng, penglm@ustc.edu.cn
Abstract. The creep deformation behavior of thermomechanically-treated Al
x
CrMnFeCoNi
(x=0.4 and 0.6) high-entropy alloys was investigated under constant tensile loadings at 600-
700 °C. It was found that the two alloys exhibited a mixed structure of fcc+bcc solid solutions
with equiaxed grains and higher Al content produced a larger volume fraction of bcc phase.
The double logarithmic plot of creep rate versus applied stress in Al
0.4
CrMnFeCoNi alloy was
divided into two distinct regions with different stress exponents and activation energies
depending on testing temperature and applied stress. In contrast, the Al
0.6
CrMnFeCoNi alloy
exhibited a single value of stress exponent without transitional features. The
Al
0.6
CrMnFeCoNi alloy showed lower creep resistance at a given stress and testing
temperature compared to Al
0.4
CrMnFeCoNi alloy, which was ascribed to the higher stacking
fault energy in the former alloy.
1. Introduction
Unlike traditional alloys that have one or two major metal elements, multi-component alloys or high
entropy alloys (HEAs) contain at least five major elements with concentration range of 5-35 at% and
have been an attractive subject as promising solid solution materials [1]. HEAs exhibit excellent
mechanical properties such as excellent wear resistance, good corrosion resistance and high-
temperature softening resistance [2-6]. The intrinsic mechanisms such as sluggish diffusion and
lattice distortion for HEAs suggest an effective heat-resistance during plastic deformation process,
especially under high-temperature performance. The single-fcc structured CoCrFeMnNi alloy has
been widely investigated due to its excellent thermodynamic stability and high toughness especially
in extreme low temperature [7]. Nevertheless, its strength and creep resistance needs to be further
improved. One of the effective approaches is to introduce a certain amount of bcc solid solution into
this alloy via adding other metal elements to obtain dual-phased microstructures. With this concept in
mind, (FeCoNiCrMn)
100-x
Al
x
alloys were recently developed and their tensile properties in cast state
were investigated at room temperature to examine the influence of microstructures [8].
Unfortunately, quite few reports have been available up to date on the high temperature creep
deformation of these alloys.
Accordingly, the aim of present study is to investigate the tensile creep behavior of
Al
x
CrMnFeCoNi high-entropy alloys. The stress exponent and activation energy for creep were
Cao, C., Xu, J., Hao, Y., Tong, W. and Peng, L.
High-Temperature Creep Behavior of AlxCrMnFeCoNi High-Entropy Alloys.
In Proceedings of the International Workshop on Materials, Chemistry and Engineering (IWMCE 2018), pages 71-76
ISBN: 978-989-758-346-9
Copyright © 2018 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
71
estimated based on the power-law creep equation to examine the corresponding mechanisms
responsible for the creep deformation. The activation volume was determined to evaluate the effect
of Al on the creep resistance and the creep fracture models were discussed based on SEM
observations.
2. Experimental
The cast ingots with the nominal compositions of Al
x
CrMnFeCoNi (x=0.4 and 0.6 in molar ratio)
were fabricated by vacuum-induction melting a mixture of six pure metals (all in high purity of 99.9
wt%) in a water-cooled copper crucible. All ingots were melted at least five times to ensure chemical
homogeneity, and were drop-casted into a steel mold. The cast alloys were homogenized at 1150 °C
for 12 h in argon gas, and then rolled at 950 °C to obtain a thickness reduction of 40%. The rolled
plates were finally recrystallized at 1100 °C for 2 h. Dog-bone shaped specimens with a gauge length
of 25 mm were cut by electrical discharge machining. The tensile samples were tested on the CSS-
3905 multi-functional machine under different loadings.
The crystalline structures of the samples were identified by X-ray diffraction (XRD) (PANalytical
X’Pert Pro) with Cu-K
α
radiation at 40 kV. The microstructures were observed using an optical
microscope (OM) (AxioImager.Alm) and phase-compositional analysis were conducted using an
energy dispersive spectroscope (EDS). For microstructural observation, samples were initially
polished to 2500-grit SiC paper and, subsequently, etched with a solution composed of hydrogen
peroxide and hydrochloric acid.
3. Results and discussions
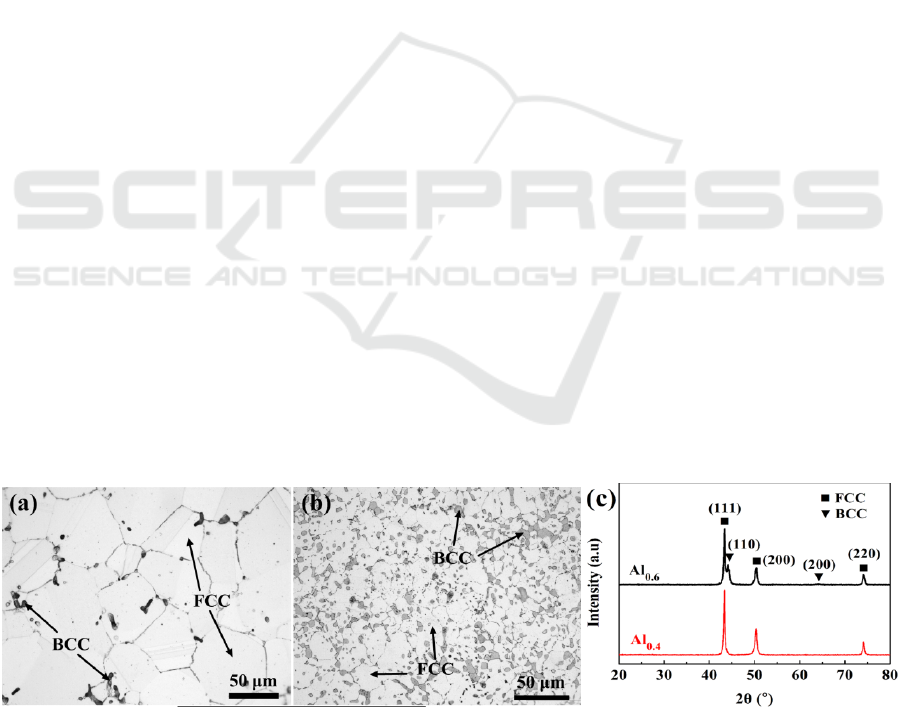
3.1. Microstructures and XRD diffraction analysis
Figure 1 shows the microstructures and XRD patterns of Al
x
CrMnFeCoNi alloys (subsequently
denoted as Al
x
where x=0.4 or 0.6). Typical equiaxed grains and annealing twins are observed in the
morphologies of these alloys. The bright and dark regions located at grain boundaries are identified
as fcc and bcc phases, respectively. The volume fraction of bcc phase and the corresponding (110)
bcc
peak increase with the increase of Al concentration. The chemical compositions of phases measured
by EDS are summarized in the Table 1. It is worth noting that Mn is homogeneously distributed in
both fcc and bcc phases and bcc phase is enriched in Al and Ni, which is attributed to the mixing
effect of enthalpies. The enthalpies of Cr-, Mn-, Fe-, Co- and Ni-Al are -10, -19, -11, -19 and -22
kJ/mol [9], respectively. Accordingly, Ni, not Cr and Fe tend to diffuse to bond with Al, leading to
the formation of Ni-Al-rich bcc phase. In addition, the grain size of fcc phase for Al
0.6
is smaller
compared to that of Al
0.4
as the grain-boundary bcc phases can hinder the growth of fcc phase.
Figure 1. Microstructures of (a) Al
0.4
CrMnFeCoNi alloy and (b) Al
0.6
CrMnFeCoNi alloy, and (c)
XRD patterns.
IWMCE 2018 - International Workshop on Materials, Chemistry and Engineering
72
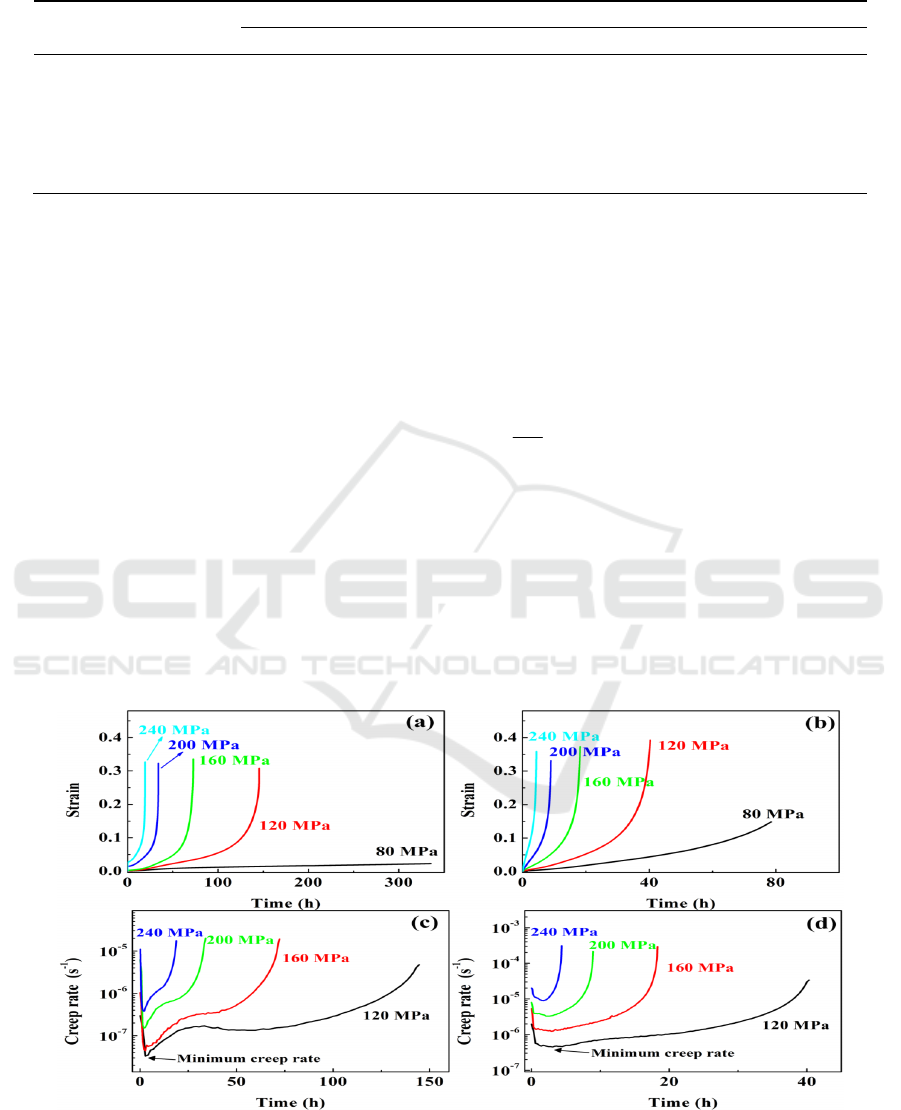
Table 1. Chemical compositions of Al
x
HEAs obtained by EDS.
Alloys Crystal Chemical compositions/at. %
Al C
r
Mn Fe Co Ni
Al
0.4
Overall 6.42 19.50 18.72 18.81 17.58 18.97
FCC 6.05 19.92 18.64 19.55 17.65 18.34
BCC 22.95 6.00 18.15 7.41 12.02 33.48
Al
0.6
Overall 9.71 18.70 17.86 17.97 17.82 17.93
FCC 6.89 20.81 18.06 20.00 18.66 15.59
BCC 24.12 7.09 16.62 8.15 14.71 29.33
3.2 Creep deformation
Figure 2 exhibits the variations of creep strain and creep rate with testing temperature of Al
0.4
and
Al
0.6
alloys at 650 ºC. It is found that Al
0.6
shows a higher creep rate than Al
0.4
at a given temperature
and stress. The creep deformation at the minimum creep rate held a short duration especially under
higher applied stresses. In this case, the minimum creep rate is taken as the steady-state creep rate
ε
&
,
and usually used to evaluate the creep behavior by correlating with the applied stress
σ
based on the
Arrhenius-type equation [10]:
)exp(
R
T
Q
A
n
−=
σε
&
(1)
Where
A
is a material-dependent constant,
σ
is the applied stress,
n
is the stress exponent, and
Q
is the
activation energy for creep deformation. As shown in Figure 3, the stress exponents were determined
by plotting the variation of the steady-state creep rate with the applied stress on double logarithmic
scales. It is found that the exponents of Al
0.4
at 650 ºC and 700 ºC exhibit a stress-dependent
transition with two distinct stress regions, i.e. low-stress region I with
n =1.6-2.2 and high-stress
region II with
n
=4.2-4.9, indicating a transition of rate-controlling mechanisms. In contrast, Al
0.6
shows no transition in the stress exponents, with a single value of 5.8 at 600 and 2.8-3.5 at 650-700
ºC.
Figure 2. Creep curves at 650 ºC for (a) Al
0.4
and (b) Al
0.6
alloys, and creep rate curves at 650 ºC for
(c) Al
0.4
and (d) Al
0.6
alloys at selected loadings.
High-Temperature Creep Behavior of AlxCrMnFeCoNi High-Entropy Alloys
73
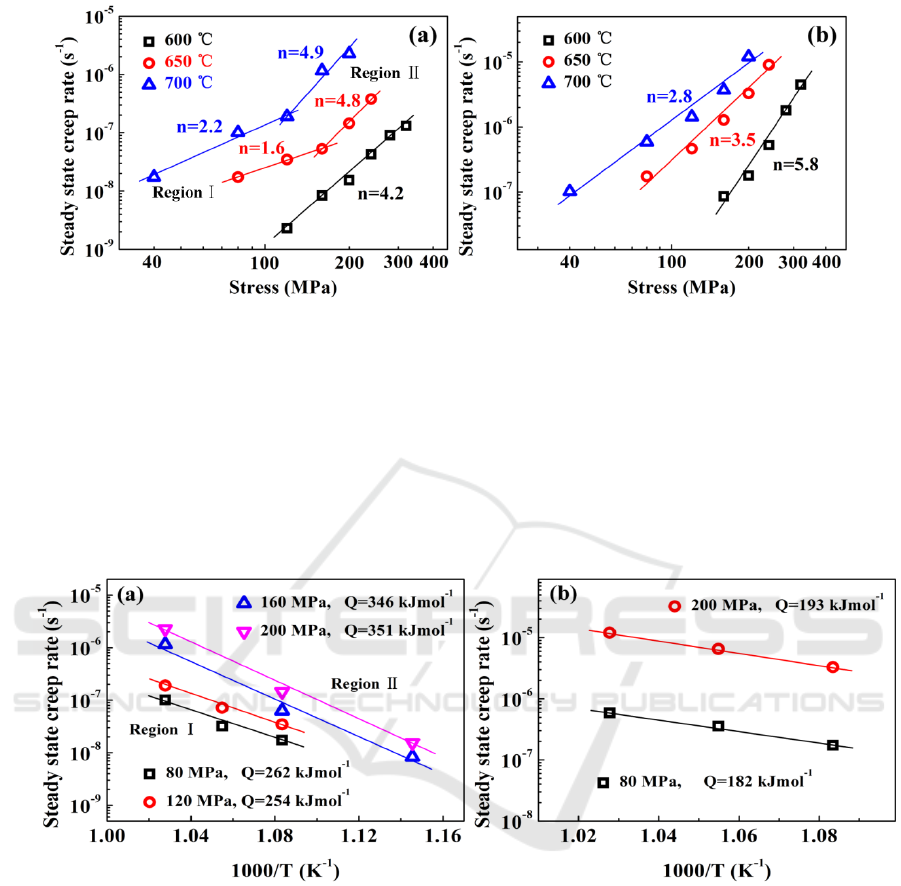
Figure 3. Steady state creep rate as a function of the applied stress on a double logarithmic plot for
(a) Al
0.4
and (b) Al
0.6
alloys.
Based on equation (1), the activation energy was estimated by plotting the steady-state creep rate
against
1/T
at constant stresses on a semi-logarithmic scale as depicted in Figure 4. The average
values of activation energy for region I and II are calculated as 258 and 349 kJ mol
-1
, respectively,
which are close to that of steady-state flow of FeCoNiCrMn alloy (
284–333 kJmol
-1
) [10]. In
contrast, the average activation energy at 650-700 ºC is 188 kJ mol
-1
.
Figure 4. Arrhenius plot of steady-state creep rate versus temperature to determine the activation
energy of (a) Al
0.4
CrMnFeCoNi alloy and (b) Al
0.6
CrMnFeCoNi alloy.
In general, it is difficult to determine which element is the contributing solute atom in the HEAs
and the activation energies for lattice diffusion of constituent elements are not available. The
activation energy of Al element is 126 kJ mol
-1
, which is much lower than those for lattice diffusion
of other constituent elements in the CrMnFeCoNi alloy (288–317 kJ mol
-1
) [11,12]. Thus, it is
reasonable to deduce that the creep deformation in these alloys is controlled by the five major
elements with high activation energy. For Al
0.4
alloy, the creep mechanism of low-stress region is
ascribed to the viscous glide of dislocations, which coincides with the previous research on the
deformation mechanism with the stress exponent of 2 in FeCoNiCrMn alloy [10]. The activation
energy of 258 kJ mol
-1
is slightly lower than that of lattice diffusion of constituent elements in the
CrMnFeCoNi alloy, suggesting that the creep rate is controlled by the diffusion of constituent
elements as the solute atoms. In the region II, the stress exponent is close to 5, suggesting a
IWMCE 2018 - International Workshop on Materials, Chemistry and Engineering
74

dislocation-climb mechanism. The larger activation energy of region II compared to that of region I
is attributed to more constituent elements involved in solute atmosphere with the increase of stresses.
The activation energy of 349 kJ mol
-1
for region II is close to that of lattice diffusion of Ni (317.5 kJ
mol
-1
), indicating that the creep rate is probably controlled by the sluggish diffusing species such as
Ni. However, for Al
0.6
tested under 650-700 ºC, the activation energy of 188 kJ mol
-1
is much lower
than that of major constituent elements. It is reported that the activation energy of pipe diffusion is a
value around 0.4-0.7 times of the activation energy of lattice diffusion [13]. Considering the stress
exponent of ~3, it can be inferred that the viscous glide of dislocations controlled by pipe diffusion is
responsible for the creep mechanism in this region.
It is well known that the activation volume
*
V
as a common parameter can be employed to
analyze the deformation mechanism. This parameter is defined as [14]
σ
ε
∂
∂
=
&
ln
*
RTV
(2)
where R represents the gas constant. The
*
V values for Al
0.4
are calculated as 1.40×10
-4
, 1.48×10
-4
and 2.03×10
-4
m
3
mol
-1
for 600 ºC, 650 ºC and 700 ºC, respectively. For Al
0.6
,
*
V takes corresponding
values of 1.80×10
-4
, 1.89×10
-4
and 2.41×10
-4
m
3
mol
-1
. As a result, higher activation volume in the
Al
0.6
alloy causes a higher creep rate compared to Al
0.4
under identical testing conditions. Similar
feature have been verified in the previous investigation of the stress relaxation test on Al
x
CoCrFeNi
alloys [14].
3.3. Fractographs
Fracture morphologies of the two HEAs after creep rupture were presented in Figure 5. It was
observed that the fractographs of Al
0.4
exhibited typically ductile fracture with obvious dimples,
whereas the fractographs of Al
0.6
revealed a quasi-cleavage fracture mode characterized by cleavage
surfaces and tearing ridges. It can be also noted that more oxides appear on the fracture surfaces
subjected to long-term exposure at 700 ºC.
Figure 5. Fractographs of Al
0.4
CrMnFeCoNi alloy crept at (a) 600 ºC and (b) 700 ºC, and
Al
0.6
CrMnFeCoNi alloy at (c) 600 ºC and (d) 700 ºC.
High-Temperature Creep Behavior of AlxCrMnFeCoNi High-Entropy Alloys
75

4. Conclusions
Two alloys showed equiaxed grains with fcc+bcc duplex structure, and higher Al content yields more
volume of bcc phase and finer microstructure. Two creep-deformation regions for Al
0.4
alloy are
observed. In the low-stress region, the stress exponent is about 2 and the activation energy is 258 kJ
mol
-1
. The lattice diffusion controlled dislocation-glide mechanism is operative for the creep
deformation. In contrast, the stress exponent of ~5 and the activation energy of 348 kJ mol
-1
indicate
that lattice diffusion of sluggish diffusing species controlled dislocation-climb is responsible for the
creep mechanism in the high-stress region. In the Al
0.6
alloy, stress exponents are 5.8 at 600 °C and
3.2 at 650-700 °C. The calculated activation energy of 188 kJ mol
-1
at 650-700 °C implies that the
creep deformation was dominated by dislocation glide controlled by pipe diffusion. In addition, the
Al
0.4
CrMnFeCoNi alloy with less stacking fault energy exhibits higher creep resistance compared to
Al
0.6
CrMnFeCoNi alloy. The investigated findings are important not only for understanding the
tensile creep behavior of Al
x
CrMnFeCoNi alloys, but also for future application of HEAs in high
temperature.
Acknowledgements
This work was supported by the Natural Science Foundation of China (No. 11572306) and the
Fundamental Research Funds for Central Universities (WK2090050040).
References
[1] Yeh J W, Chen S K, Lin S J, Gan J Y, Chin T S, Shun T T, Tsau C H and Chang S Y 2004 Adv.
Eng. mater. 6 299
[2] Poletti M G, Fiore G, Gili F, Mangherini D and Battezzati L 2017 Mater. Des. 115 247
[3] Gwalani B, Ayyagari A V, Choudhuri D, Scharf T, Mukherjee S, Gibson M and Banerjee R
2018 Mater. Chem. Phys. 210 197
[4] Lin C M and Tsai H L 2011 Intermetall. 19 288
[5] Chokshi A H 2018 Mater. Chem. Phys. 210 152
[6] He J Y, Wang H, Wu Y, Liu X J, Nieh T G and Lu Z P 2017 Mater. Sci. Eng. A. 686 34
[7] Otto F, Dlouhý A, Somsen C, Bei H, Eggeler G and George E P 2013 Acta Mater. 61 5743
[8] He J Y, Liu W H, Wang H, Wu Y, Liu X J, Nieh T G and Lu Z P 2014 Acta Mater. 62 105
[9] Takeuchi A and Inoue A 2005 Mater. Trans. 46 2817
[10] He J Y, Zhu C, Zhou D Q, Liu W H, Nieh T G and Lu Z P 2014 Intermetall. 55 9
[11] Edalati K and Horita Z 2011 Scr. Mater. 64 161
[12] Tsai K Y, Tsai M H and Yeh J W 2013 Acta Mater. 61 4887
[13] Fu J X, Cao C M, Tong W, Hao Y X and Peng L M 2017 Mater. Sci. Eng. A. 690 418
[14] Cao T, Shang J, Zhao J, Cheng C, Wang R and Wang H 2016 Mater. Lett. 164 344
IWMCE 2018 - International Workshop on Materials, Chemistry and Engineering
76
