
Synthesis of SAPO-34 Zeolite with Different Template Agents
and DTO Catalytic Studies
L Yang, S C Zhang, Y P Feng, Z T Zhu, Y B Song and Y W Fang
*
Department of Chemistry, College of Science, Shantou University, Shantou,
Guangdong, 515063, P. R. China.
Corresponding author and e-mail: Y W Fang, ywfang@stu.edu.cn
Abstract. SAPO-34 molecular sieves were synthesized by using different templates of
triethylamine, morpholine and tetraethylammoniu m hydroxide under hydrothermal conditions.
Phase purity and crystal mo rphology of the synthesized samples were characterized by XRD
and SEM. The catalytic test of dimethyl ether to olefins (DTO) over all the synthesized
samples were studied. The results of catalytic activity test showed that the TEAOH-SAPO-34
zeolite catalysts with smaller crystal size exhib ited excellent catalytic performance compared
to TEA-SAPO-34 and MOR-SAPO-34 catalysts.
1. Introduction
Zeolites as catalysts play an important role in petrochemical industry. Previous studies show that
ZSM-5 zeolite with MFI structure and SAPO-34 zeolites with CHA structure exhibited excellent
catalytic performance in methanol to olefins (MTO) or dimethyl ether to olefins (DTO) processes [1-
3]. Especially, SAPO-34 zeolites with a large CHA cage and 8-ring pore opening have good
selectivity for light olefins [4, 5]. However, the SAPO-34 zeolite catalysts face the problem of rapid
deactivation in the process of reaction because of the formation of coke.
Previous research indicates that decreasing the crystal size [6, 7], forming thin slice structure or
introduction of the hierarchical porous structure could effectively enhance mass transfer and reduce
the rate of coking, thus prolong the catalyst lifetimes of SAPO-34 zeolite catalysts [8-10]. As we
have known, template agents have a great influence on the morphology of the synthesized SAPO-34
zeolite, and the crystal size of the synthesized SAPO-34 zeolite is also very different.
So far more than 20 types of templates have been utilized to synthesize SAPO-34 catalysts.
Among these templates, tetraethylammonium hydroxide (TEAOH), morpholine (MOR) and
triethylamine (TEA) are the most commonly used templates. The choice of templates significantly
impacts the particle sizes and then affects the physical chemical properties of zeolites [11].
He et al. studied that adjusting the crystallite size of SAPO-34 molecular sieve by the dual
template method [12]. They found the size of SAPO-34 zeolite synthesized by using TEA as template
is larger and there are more centres of strong acid. The crystals size of SAPO-34 zeolite was
decreased when two templates of TEA and TEAOH were used. They concluded that TEAOH
template was beneficial to the formation of SAPO-34 zeolites with small crystal size.
Sun et al. successfully prepared nanosheet-like SAPO-34 molecular sieves with different silicon
contents under conventional hydrothermal condition using tetraethylammonium hydroxide as the
Yang, L., Zhang, S., Feng, Y., Zhu, Z., Song, Y. and Fang, Y.
Synthesis of SAPO-34 Zeolite with Different Template Agents and DTO Catalytic Studies.
In Proceedings of the International Workshop on Materials, Chemistr y and Engineering (IWMCE 2018), pages 545-551
ISBN: 978-989-758-346-9
Copyright © 2018 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
545

template [13]. They found the SAPO-34 zeolites with nanoplate structure provide the shortest
diffusion length for reactant and products and effectively reduce the coke formation rate, thus
prolong the lifetimes of SAPO-34 catalysts.
In this work, we investigated the effect of different template agents on the synthesis of SAPO-34
molecular sieves. We used TEA, MOR and TEAOH as templates to synthesize SAPO-34 molecular
sieves. The XRD and SEM show that using different templates could synthesis SAPO-34 catalysis
with different crystal size and morphology. Compared with the TEA-SAPO-34 and MOR- SAPO-34
zeolite catalysts, TEAOH-SAPO-34 crystals show smaller crystal size. In the process of DTO
catalytic reaction, the TEAOH-SAPO-34 zeolite catalysts exhibit the longest catalyst life and the
highest total selectivity of ethylene and propylene.
2. Experiments
2.1. Material
All the reagents used were Aluminium isopropoxide (AIP, 99% ), Tetraethyl orthosilicate ( TEOS,
99% ), Phosphoric acid ( 85 wt% ), Morpholine ( MOR, 99% ), Triethylamine(TEA, 99%),
Tetraethyl ammonium hydroxide ( TEAOH, 25% ).
2.2. Preparation of catalysis.
TEA-SAPO-34 zeolite catalysts were synthesized by using the template of TEA with molar ratios of
raw materials: 1.0Al
2
O
3
:1.0P
2
O
5
:0.4SiO
2
:4.5TEA:70H
2
O under the hydrothermal conditions.
Typically, 2.04 g aluminium isopropoxide, 0.615 ml phosphoric acid and 7.2 ml deionized water
were mixed into a beaker, which were stirred for 20 minutes at the temperature of 35
o
C. After that,
adds 3.29 ml TEA and continuous stirring for 2 hours. Finally, 0.452 ml TEOS was added into the
mixture. The mixture colloidal solution was stirred for 4 h and then was transferred into Teflonlined
autoclave, crystallized at 180
o
C for 48 h. After crystallization, the solid products were separated and
washed with deionized water several times, followed dried at 80
o
C. Finally, the dried products were
calcined at 550
o
C for 6 h with the heating rate of 2
o
C per min.
MOR-SAPO-34 zeolite catalysts were synthesized by using the template of MOR with molar
ratios of raw materials: 1.0Al
2
O
3
:1.0P
2
O
5
:0.6SiO
2
:3.0MOR:70H
2
O under the hydrothermal
conditions. Typically, 2.04 g aluminium isopropoxide, 0.615 ml phosphoric acid and 7.2 ml
deionized water were mixed into a beaker, which were stirred for 20 minutes at the temperature of
35
o
C. After that, adds 1.32 ml MOR and continuous stirring for 2 hours. Finally, 0.677 ml TEOS was
added into the mixture. The mixture colloidal solution was stirred for 4 h and then was transferred
into Teflonlined autoclave, crystallized at 180
o
C for 48 h. After crystallization, the solid products
were separated and washed with deionized water several times, followed dried at 80
o
C. Finally, the
dried products were calcined at 550
o
C for 6 h with the heating rate of 2
o
C per min.
TEAOH-SAPO-34 zeolite catalysts were synthesized by using the template of TEAOH with
molar ratios of raw materials: 1.0Al
2
O
3
:1.2P
2
O
5
:0.5SiO
2
:2.0TEAOH:70H
2
O under the hydrothermal
conditions. Typically, 2.04 g aluminium isopropoxide, 0.738 ml phosphoric acid and 7.2 ml
deionized water were mixed into a beaker, which were stirred for 20 minutes at the temperature of
35
o
C. After that, adds 5.66 ml TEAOH and continuous stirring for 2 hours. Finally, 0.564 ml TEOS
was added into the mixture. The mixture colloidal solution was stirred for 4 h and then was
transferred into Teflonlined autoclave, crystallized at 180
o
C for 48 h. After crystallization, the solid
products were separated and washed with deionized water several times, followed dried at 80
o
C.
Finally, the dried products were calcined at 550
o
C for 6 h with the heating rate of 2
o
C per min.
IWMCE 2018 - International Workshop on Materials, Chemistry and Engineering
546
2.3 Characterization
The phase purity and crystallinity of the samples were characterized by powder X-ray diffraction
(XRD) with Cu Kα radiation. The crystal size and morphology of the samples were observed by
scanning electron microscopy (SEM) using a JSM-6360LA electron microscopy
2.4 Catalytic activity test
The catalytic activity test of the samples for the DTO reaction was performed in a quartz tubular
fixed-bed reactor under atmospheric pressure. The catalyst (300 mg, 40-60 mesh) loaded in the
middle of the quartz tubular reactor was activated at 450
o
C in a N
2
flow of 40 mL per minute for 2 h
before reaction. After that, the temperature was adjusted to the reaction temperature of 400
o
C and
then the DTO reaction was starting with the flow of dimethyl ether was 7.5 mL per minute. The
reaction products were analysed using on-line gas chromatograph (Agilent GC 7820), equipped with
a FID detector and Plot-Q column (HP-PLOT/Q, 19095P-Q04, 30 m×530 μm×40μm).
3. Results and discussion
3.1. XRD characterization results
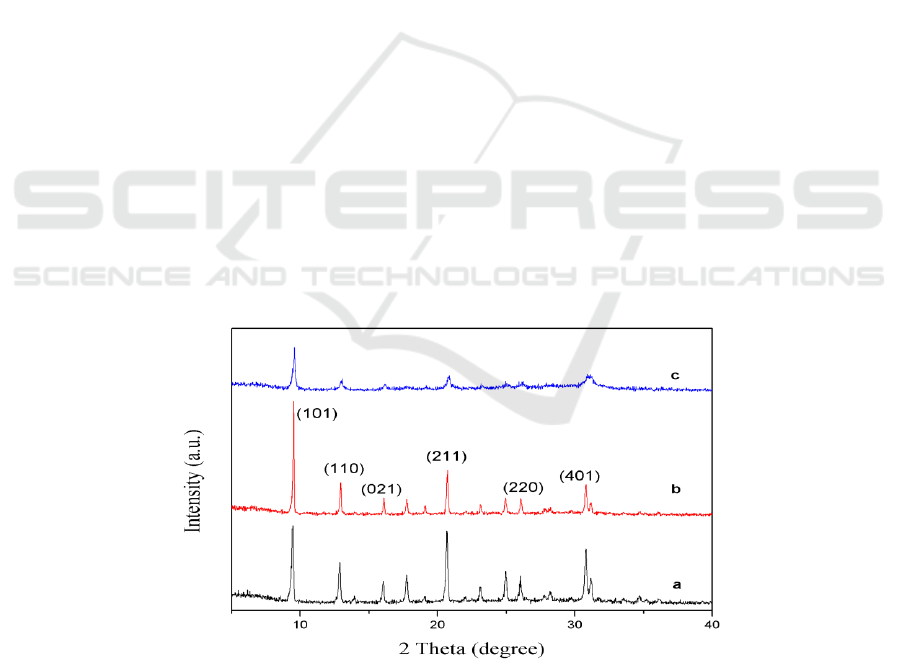
The X-ray diffraction patterns of SAPO-34 molecular sieves synthesized by different templates are
shown in Figure 1. From the results we can know, all the samples show the typical diffraction peaks
of the CHA structure, where 2 theta at 9.5°, 12.5°, 16.6°, 20.5°, 26° and 31.2° corresponding to (101),
(110), (021), (211), (220) and (401) planes respectively, which proved that all the samples have the
phase purity of SAPO-34 zeolite [14]. Besides, the characteristic diffraction peaks at 26° and 31.2°
are double peaks, which is consistent with others references. We can observe that the characteristic
diffraction peak of MOR-SAPO-34 molecular sieve is stronger than the characteristic diffraction
peak of TEA-SAPO-34 and TEAOH-SAPO-34 molecular sieve, which indicates that the samples
synthesized by using MOR as template had higher crystallinity. Compared with the TEA-SAPO-34
and MOR-SAPO-34 molecular sieves, the characteristic diffraction peaks of TEAOH-SAPO-34
molecular sieves are obviously broadened, which indicates that the crystal size of TEAOH-SAPO-34
molecular sieves is decreased [15].
Figure 1. XRD patterns of SAPO-34 zeolite synthesized by different templates: (a) TEA-SAPO-34,
(b) MOR-SAPO-34 and (c) TEAOH-SAPO-34.
3.2. SEM characterization results
Figure 2 shows the SEM images of the synthesized SAPO-34 by different templates. The TEA-
SAPO-34 zeolites show the characteristic cubic-like morphology with average particle size about 3-
Synthesis of SAPO-34 Zeolite with Different Template Agents and DTO Catalytic Studies
547

5μm, where we can observe in the SEM images of a and b. The SEM images of c and d are the
MOR-SAPO-34 crystals. Compared with the TEA-SAPO-34 crystals, the MOR-SAPO-34 crystals
have the same cubic-like morphology and higher crystallinity, the average crystal size is about 1-2
um. From the SEM images of e and f, we know that the TEAOH-SAPO-34 zeolites show the
smallest crystal which exhibits the Nano-sheets structure with approximately the crystal size of
500×400×200 nm. The crystal size of the TEAOH-SAPO-34 zeolites is decreased, which is
consistent with the result of XRD.
Figure 2. Synthesized SAPO-34 by different templates: MOR-SAPO-34(a, b), TEA-SAPO-34 (c, d)
and TEAOH-SAPO-34 (e, f).
3.3. Activity test result of DTO
Activity test of dimethyl ether conversion were performed in a fixed bed reaction at 400
o
C over the
synthesized SAPO-34 catalysts by different templates. Figure 3(a) shows the conversion of DME
with time–on-stream (TOS) over the prepared samples. From the results we can know that all the
samples exhibit high catalytic activity, where the conversion of DME is up 100%. We defined the
deactivation of catalysts when the conversion of DME was less than 100%. Different samples show
IWMCE 2018 - International Workshop on Materials, Chemistry and Engineering
548

the different lifetime of catalysts, where the catalyst lifetime of TEA-SAPO-34, MOR- SAPO-34 and
TEAOH-SAPO-34 catalysts is 168 min, 226 min and 302 min respectively. The TEAOH-SAPO-34
catalysts show the longest lifetimes compared with the TEA-SAPO-34 catalysts and MOR- SAPO-34
catalysts. Meanwhile, ethylene and propylene are the main reaction products and the total maximum
selectivity can reach 75.6%-80.2% we could observe in Figure 3(b). TEAOH-SAPO-34 zeolites with
the Nano-sheets structure show the longest catalyst lifetimes (302 min) and the highest selectivity of
ethylene and propylene (80.2%), which can attribute to the smaller crystal size, which can shorten
diffusion distance and enhance mass transfer [16].
Figure 3. (a)DME conversion variation with time-on-stream and (b) selectivity of C
2
H
4
and C
3
H
6
variation with time-on-stream over the synthesized SAPO-34 by different templates: TEA, MOR and
TEAOH. Reaction conditions: WHSV = 2.5 h
-1
, T = 400
o
C, catalyst weight = 300 mg.
After the reactions, all the deactivated catalysts were evaluated by thermal analysis. Figure 4
shows the TG curves of the deactivated catalysts of all the samples. The weight loss from the
combustion of the retained coke species are 10.02%, 12.08% and 13.59% for TEA-SAPO-34, MOR-
SAPO-34 and TEAOH-SAPO-34 catalysts, respectively (Table 1.). Compared with Figure 3(a), the
deactivation occurs at different time-on-stream, the coking rate is different for these catalysts, and the
detailed data is summarized in Table 1. The TEAOH-SAPO-34 catalysts with Nano-sheet
morphology shows the best catalyst performance but the TEA-SAPO-34 catalysts with cubic-like
morphology and large crystals shows the worst catalyst performance, which can be attributed to the
difference of crystal size. In the process of catalytic reactions the Nano-sheets structure not only
could shorten the mass transfer distance and greatly improve diffusion efficiency of reactants and
products but also could reducing the coking rates, thus prolong the catalyst lifetime [17].
4. Conclusions
In this work, we explored the influence of different template agents on the synthesis of SAPO-34
molecular sieves. We used TEA, MOR and TEAOH as template to synthesis SAPO-34 zeolites and
compare the crystal size, morphology and catalytic performance of the SAPO-34 catalysis. The
results showed that the TEAOH-SAPO-34 zeolite with the Nano-sheets structure has smaller crystal
size compared to the TEA-SAPO-34 and MOR- SAPO-34 zeolite, and showed the higher catalytic
performance, which the conversion rate of DME can be reached 100% and the selectivity of ethylene
Synthesis of SAPO-34 Zeolite with Different Template Agents and DTO Catalytic Studies
549

and propylene can be reached 80.2%. The smaller crystal sized catalysts not only could shorten the
mass transfer distance and greatly improve diffusion efficiency of reactants and products but also
could reducing the coking rates, thus prolong the catalyst lifetime. Therefore, it is of greatly
significant to explore the synthesis of Nano sized SAPO-34 molecular sieves.
Table 1. Coke analysis in the DTO reaction of the synthesized SAPO-34 by different templates: TEA,
MOR and TEAOH.
Catalyst
TEA-SAPO-34
MOR-SAPO-34
TEAOH-SAPO-34
Coke ( %, g / g
cat
)
10.02
12.08
13.59
TOS ( min )
168
226
302
R
coke
(mg / min)
a
0.178
0.160
0.135
a
R
coke
(mg / min) = coke amount (mg) / reaction time (min).
Figure 4. The TG curves of the deactivated catalysts of the synthesized SAPO-34 by different
templates: TEA, MOR and TEAOH.
Acknowledgement
The work was supported by the National Fund Cultivation Project (NFC 15001), the Science and
technology planning project of Guangdong Province (Nos. 2012CXZD0024, 2013KJCX0081and
2014A020216045).
References
[1] Chang C D 1984 Catalysis Reviews-Science and Engineering 26 323-345
[2] Valtchev V and Tosheva L 2013 Chemical Reviews 113 6734-6760
[3] Tian P, Wei Y X, Ye M and Liu Z 2015 ACS Catalysis 5 1922-38
[4] Xi D, Sun Q, Xu J, Cho H, Asahina S, Li Y, Deng F, Terasaki O and Yu J 2014 J. Mater.
Chem. A. 2 17994-04
[5] Wei Y, Li J, Yuan C, Xu S, Zhou Y, Chen J, Wang Q, Zhang Q and Liu Z 2012 Chemical
Communications 48 3082-84
IWMCE 2018 - International Workshop on Materials, Chemistry and Engineering
550

[6] Wen Z, Wang C, Wei J, Sun J, Guo L, Ge Q and Xu H 2016 Catal. Sci. Technol. 6 8089-97
[7] Cheng K, Gu B, Liu X, Kang J, Zhang Q and Wang Y 2016 Angew. Chem. Int. Ed. 55 4725-28
[8] Yang H, Liu X, Lu G and Wang Y 2016 Micropor. Mesopor. Mat. 225 144-153
[9] Schulz H 2010 Catalysis Today 154 183-194
[10] Sun Q, Wang N, Xi D, Yang M and Yu J 2014 Chemical Communications 50 6502-05
[11] Sun Q, Xie Z and Yu J 2017 Natl. Sci. Rev. 0 1-17
[12] He C, Liu Z, Yang L and Cai G 1994 J. Mol. Catal. A. 8 207-212
[13] Sun Q, Ma Y, Wang N, Li X, Xi D, Xu J, Deng F, Yoon K, Oleynikov P and Terasaki O 2014
J. Mater. Chem. A. 2 17828-39
[14] Wei Y, Li J, Yuan C, Xu S, Zhou Y, Chen J, Wang Q, Zhang Q and Liu Z 2012 Chemical
Communications 48 3082-84
[15] Meng X and Xiao F 2014 Chem. Rev. 114 1521-43
[16] Sun Q, Wang N, Guo G, Chen X and Yu J 2015 J. Mater. Chem. A. 3 19783-89
[17] Li J, Wei Y, Chen J, Xu S, Tian P, Yang X, Li B, Wang J and Liu Z 2015 ACS Catalysis 5
661-665
Synthesis of SAPO-34 Zeolite with Different Template Agents and DTO Catalytic Studies
551
