
Correlation of Parasite Density with Plasma Level of TNF-α and IL-
10 in Patients Infected by Plasmodium Vivax in East Sumba District,
East Nusa Tenggara Province
Frieti Vega Nela
1
, Heny Arwati
2
and Yoes Prijatna Dachlan
2
1
Department of Immunology Postgraduate School Universitas Airlangga,Surabaya, East Java, Indonesia
2
Department of Parasitology, Faculty of Medicine, Universitas Airlangga,Surabaya, East Java, Indonesia
Keywords: IL-10, Parasite Density, Plasmodium vivax, TNF-α.
Abstract: Introduction. Annual parasite incidence (API) in East Nusa Tenggara Province (NTT) 2015 per 1000
population is 7.04%. However, API in each Public Health Center (Puskesmas) Sumba Island remains high.
High levels of pro-inflammatory cytokines in malaria infection, such as TNF-α is associated with severe
pathology, whereas, anti-inflammatory cytokines such as IL-10 is associated with acute malaria. The objective
of the study was to analyze correlation between parasite densities and plasma level of both cytokines in P.
vivax-infected patients in East Sumba Regency East Nusa Tenggara Province. Methods. Parasite densities
were calculated per 500 leucocytes on Giemsa-stained thick blood smears. The levels of TNF-α and IL-10
were measured by Enzyme-Linked Immunosorbent Assay (ELISA) method. Statistical analyses were done
by Spearman test. Results. Correlation was observed significantly in parasite density and TNF-α p = 0.032
and parasite density and IL-10 p=0.000. This result indicated that the stage of immunity in patients was not
affected by the parasite density but clinical symptoms may have a greater role in increasing and decreasing
the plasma level of cytokines. Conclusion. There was correlation between parasite densities and plasma level
of TNF-α and IL-10 in P. vivax infected patients is the studied areas.
1 INTRODUCTION
Malaria incidence was still high in the eastern parts of
Indonesia including Papua Province, West Papua,
East Nusa Tenggara (NTT), Central Sulawesi and
Maluku (Kemenkes, 2013). During 2015 the Annual
parasite incidence (API) in East Nusa Tenggara
Province (NTT) per 1000 population was 7.04%, the
number of cases of positive malaria as high as 36,039
from 5,120,061 inhabitants. The API in each Public
Health Center (Puskesmas) in Sumba Island remains
high (Pusdatin, 2016).
Malaria has been known since 3,000 years ago
and is caused by protozoa of the genus Plasmodium
and transmitted by female Anopheles mosquitoes
(Gunawan, 2000). There are 5 species of parasite
causing malaria in humans, namely Plasmodium
falciparum, Plasmodium vivax, Plasmodium
malariae, Plasmodium ovale and Plasmodium
knowlesi (White et al., 2014).
Plasmodium vivax has a longer incubation time
(12 days to several months), has a erythrocyte cycle
42-48 hours and produces fewer merozoites per
schizon. It is generally known that P.vivax requires a
duffy antigen as a receptor needed to invade host
erythrocytes. In humans who do not have this antigen,
they will become resistant to the infection (Andrade
et al., 2010).
An immune response to malaria leads to parasite
elimination or persistent responses are mediated by
cytokines that cause immunopathology. In malarial
infection high levels of pro-inflammatory cytokines,
such as Tumor Necrosis Factor (TNF), Interferon
Gamma (IFN-γ) and Interleukin-6 (IL-6) are
associated with severe pathology whereas cytokines,
anti-inflammatory agents such as Transforming
Growth Factor Beta (TGF-ß) and IL-10, are
associated with acute malaria. IL-10 cytokines have
an important role as immuno-regulators from
infections caused by Plasmodium, by neutralizing
theeffects of cytokines produced by Th1 and CD8+
cells, which are responsible for immunopathology
associated with excess cytokine production (Medina
et al., 2011).
Nela, F., Arwati, H. and Dachlan, Y.
Correlation of Parasite Density with Plasma Level of TNF-Î
´
s and IL-10 in Patients Infected by Plasmodium Vivax in East Sumba District, East Nusa Tenggara Province.
DOI: 10.5220/0007542203250328
In Proceedings of the 2nd International Conference Postgraduate School (ICPS 2018), pages 325-328
ISBN: 978-989-758-348-3
Copyright
c
2018 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
325
Activated macrophages release pro-inflammatory
cytokines such as TNF-α, IL-1 and IL-6 (Tsokonas et
al., 2002). The release of TNF-α apart from activated
macrophages can also be directly induced by the
malaria parasite and its dissolved antigen such as
malaria pigment (haemozoin) and
Glycosylphosphatidylinositol (GPI). TNF-α
indirectly inhibits parasites by increasing phagocyte
activity of monocytes (Wipasa et al., 2002; Korbel et
al., 2004).
CD4+ T cells are classified into 2 major subsets
according to the cytokine production pattern. Th1
produces IL-12, IFN-γ, and TNF-α. While Th2
produces IL-4, IL-5, IL-6, IL-10. In general, Th1 cells
are responsible for cell-mediated immunity (CMI).
The cytokine activates macrophages and other cells to
produce mediators releasing inflammatory cytokines.
Th2 cells regulate humoral immune by helping B
cells to produce antibodies. Th2 cells promote the
production of immunoglobulins. Both Th1 and Th2
cells are involved in protective immunity against
malaria in the pre-eritrocytic stages, and the balance
of cytokine production by both Th is the determining
factor of the disease (Wipasa et al., 2002).
2 MATERIAL AND METHODS
2.1 Subject of Research
The blood samples were collected from East Sumba
residents by active case detection in the villages with
high API value. Passive case detection was done by
collecting blood samples from P.vivax-infected
patients who came to Puskesmas seeking medication.
Blood samples were taken from those who meet
inclusion criteria which is P.vivax positive by rapid
diagnostic test (RDT) and microscopic examination
followed by the signed informed consent.
2.2 Screening
A screening test is used to determine the inclusion
criteria described above using 2 methods: RDT and
microscopic tests. All samples were examined using
a microscopic. RDT is used when sampling is active
in the village because there is no microscope to
support microscopic examination. Microscopic
examination is still performed after the RDT results
show a positive P.vivax.
2.3 Enzyme-Linked Immunosorbent
Assay (ELISA)
Blood collection from vein cubitis patients with P.
vivax malaria three milliliters (ml), inserted blood
into the heparin tube, centrifuge for 15 minutes at a
speed of 3000 rpm, the plasma is taken and
transferred into ependorf tube using micropipette.
The measurements of TNF-α and IL10 levels use
the Enzyme-Linked Immunosorbent Assay (ELISA)
in accordance with manufacturer protocols, with all
samples running in a single assay. The ELISA was
performed and analyzed by a single operator, and
standard curves were derived from cytokine
standards.
3 RESULT AND DISCUSSION
3.1 Parasite Density
P.vivax positive samples that have been collected are
smeared in thick drops and examined using a 1000x
magnification microscope. The formula for
calculating parasite density is as follows:
Parasite density = ∑ Parasite x 8000
500
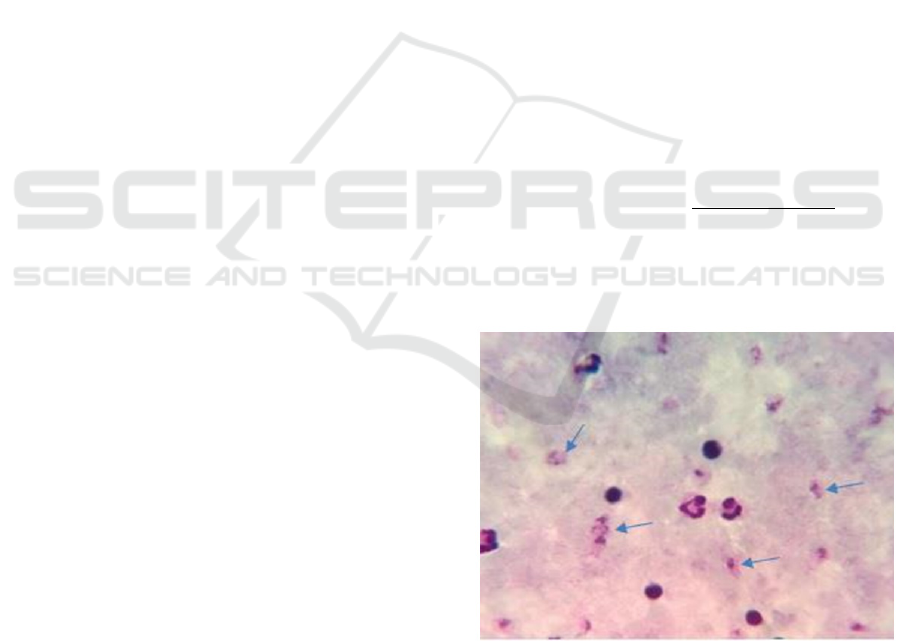
The following is a microphotography picture of
thick blood smear P.vivax:
Figure 1: Microphotography P.vivax thick blood smear
Parasite density of samples diagnosed positive
P.vivax as follows:
ICPS 2018 - 2nd International Conference Postgraduate School
326
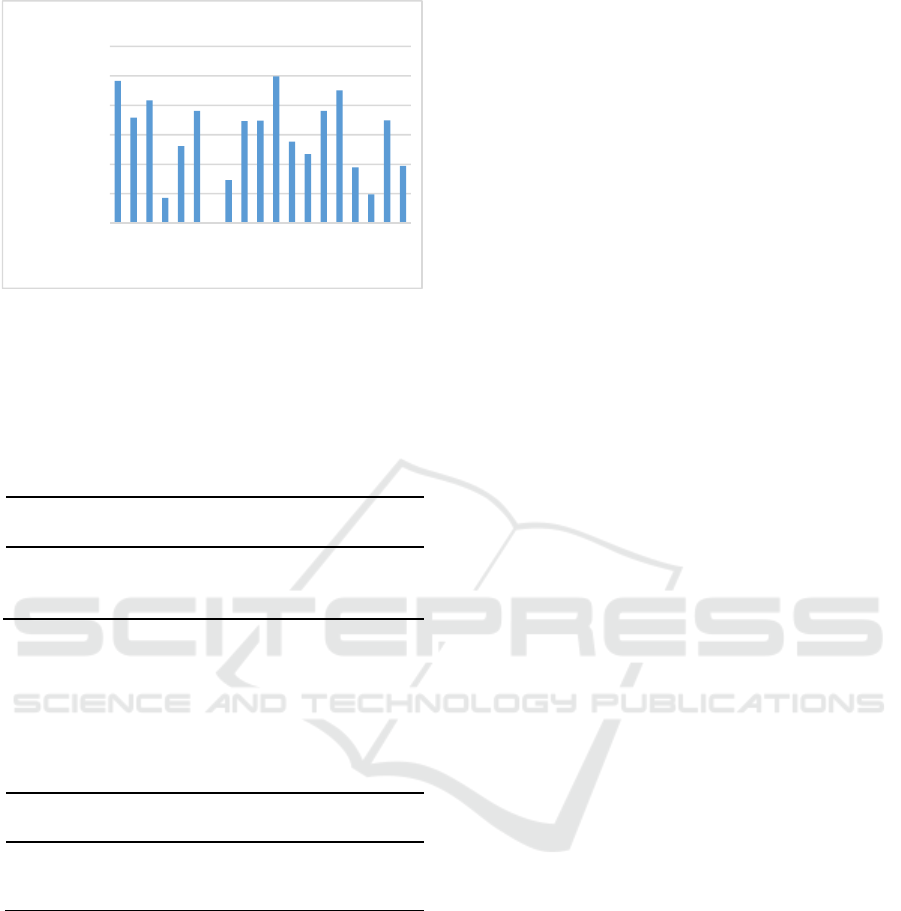
Figure 2: Parasite density P.vivax
3.2 Category of TNF- α and IL-10
Measurement of TNF-α and IL10 levels using ELISA
can be categorized according to the following:
Table 1: Category of TNF-α levels
TNF-α
Category
Frequency %
(pg
/ml
)
0
–
100
Low 9
47,37
101
–
500
Intermediate
7
36,84
> 500
High
3
15,79
TNF-α level was categorized as 3 types that is
low, intermediate, high. The percentage of TNF-α
low is 0-100 is 47.37%, intermediate 101-500 is
36.84% and high> 500 is 15.79%.
Table 2: Category of IL-10 levels
IL-10
Category
Frequency %
(pg/ml)
0
–
10
Low
11
57,89
11
–
50
Intermediate
7
36,84
> 50
Hi
g
h
1
5,26
TNF-α level was categorized as 3 types that is
low, intermediate, high. Low IL-10 percentage is 0-
10 is 57,89%, intermediate 11-50 is 36,84% and
high> 50 is 5,26%.
3.3 Category of TNF- α and IL-10
Kolgorov-Smirnov Test was used to find out the
normality of data. When the data is evenly
distributed, then Pearson test was used to analyze the
correlation between parasite density with TNF-α and
IL-10. If the data is distributed unevenly, Spearman
test was used. The correlation is significant if p <0.05
is obtained. The results showed that significant
correlation was observed significantly parasite
density and TNF-α p = 0.032 and parasite density and
IL-10 p=0.000.
3.4 Discussion
Infections caused by P.vivax have long been regarded
as benign, especially when compared with infections
caused by P.falciparum, but vivax malaria causes
more severe disease than P.falciparum infection
(Borges et al., 2013).
TNF-α is a pro-inflammatory cytokine that is the
cause of fever (Hietbink et al., 2006). At high levels
TNF-α can cause severe tissue damage (Couper et al.,
2008). At the optimum level TNF-α can kill parasites
directly, provide protection and lead to malaria
recovery. Low levels of TNF-α can inhibit the growth
of parasites in the stadium in the blood by activating
the cellular immune system (Raza et al., 2013).
IL-10 is the main anti-inflammatory cytokine in
the natural immune response and adaptive
inflammatory response through the process of
inactivation of macrophages and T cells (Dodoo et al.,
2002). High level of IL-10 will prevent the
development of severe malaria anemia (Weatherall et
al., 2002). The occurrence of severe anemia is
associated with a decrease in the concentration of IL-
10 in the circulation and increases the ratio of TNF-α
and IL-10. This condition contributes to the reversible
suppression of bone marrow activity that occurs in
malaria patients (Malaguarnera, 2002).
4 CONCLUSIONS
There was correlation between parasite densities and
plasma level of TNF-α and IL-10 in P. vivax infected
patients is the studied areas. This result indicated that
the stage of immunity in patients was not affected by
the parasite density but clinical symptoms may have
more role in increasing and decreasing the plasma
level of cytokines.
REFERENCES
Andrade BB, Antonio RF, Sebastiao MS, Jorge C, Luis
MA, Aldina B, et al. 2010. Severe Plasmodium vivax
malaria exhibits marked inflammatory
imbalance.https:malariajournal.biomedcentral.co
m/artless/10.1186/1475-2875-9-13.
Borges, I. Quessi, Cor JF Fontes and Amicar S. Damazo.
2013. Analysis of lymphocytes in patients wih
0
2.000
4.000
6.000
8.000
10.000
12.000
135791113151719
Densitas Parasit
Kode Sampel
Correlation of Parasite Density with Plasma Level of TNF-Î
´
s and IL-10 in Patients Infected by Plasmodium Vivax in East Sumba District,
East Nusa Tenggara Province
327

Plasmodium vivax malaria and its relation to the
annexim-A1and IL-10. Malaria journal. 12-455.
Couper KN, Blount DG, Riley.2008. EM: IL-10: The
master regulator of immunity to infection. J Immunol.
180:5777-7.
Dodoo D, Omer FM, Todd J., et al. 2002. Absolute levels
and ratios of proinflammatory and anti-inflammatory
cytokine production in vitro predict clinical immunity
to plasmodium falciparum malaria. The Journal of
Infectious. 185: 971– 9.
Gunawan, S. 2000. Epidemiologi malaria. Dalam:
Harijanto PN (ED) Malaria epidemiologi, patogenesis,
manifestasi klinis & penanganan. Jakarta. EGC.
Hietbink F, Koenderman I, Rijkers
GT, Leenen LPH. Trauma: the role of the innate
immune system.2006. [internet] [Disitasi 13 Agustus
2018] http://www.wjes.
Kemeterian Kesehatan RI. Hasil Riskesdas 2013.
http://terbitan.litbang.depkes.go.id/penerbitan/ind
ex.php/blp/catalog/series/rkd.
Korbel D S, Finney O C, and Riley E M. 2004. Natural
killer cells and inate immunity to protozoan pathogens.
International Journa for Parasitology 34, 1517-1528.
Raza AGN, Sarwar Zubairi AB, Raheem A, Nizami S.
2013. Beg M: Tumor necrosis factor -α, interleukin-10,
intercellular and vascular adhesion molecules are
possible biomarkers of disease severity in complicated
Plasmodium vivax isolates from Pakistan. PLoS One
8:e81363.
Malaguarnera, L., Musumeci, S. 2002. The immune
response to plasmodium falciparum malaria. Lancet
Infect Dis. 2: 472 – 8.
Medina, Tiago S., Sheyla PT., C., Maria, DO., Ana, MV.,
Jose, MS., Tassia, FG., Antonio, CR. V., Marinete MP.,
Joao, SS., Maristela, GC.2011. Increased interleukin-10
and interferon-ϒ levels in plasmodium vivax malaria
suggest a reciprocal regulation which is not altered by
IL-10 gene promoter polymorphism. Malaria Journal,
10:264.
Tsakonas K A and Riley E M .2002.Innate Immune
Reponse to Malaria : Rapid Induction of INF-γ from
Human NK Cells by Live Plasmodium falcifarum-
Infected Erythrocytes. Jurnal of Immunology
169:2956-2963.
Pusat Data Informasi Kementrian Kesehatan
RI.2016.InfoDATIN malaria ISSN 2442-7659.
Weatherall, DJ., Miller, LH. 2002. Malaria and the red cell.
The American Society of Hematology.
White. Nicholas j., Sasithon P., Tran T.H., M.Abdul Faiz,
Olugbengan A. Mokuolu. Arjen M. Dondorp. 2014.
Malaria Journal The Lance. Vol.383.
Wipasa, J,Ellot S, Xu H, and God M F. 2002. Immunity to
asexual blood stage malaria and vaccine approaches.
Immunologiy and Cell Biology, 80, 401-4004.
ICPS 2018 - 2nd International Conference Postgraduate School
328
