
The Effect Collagen to Granuloma Structure and Immune Response
on Granuloma Tuberculosis In Vitro Models
Ira Pangesti
ab1
, Agung Dwi Wahyu Widodo
2
and Jusak Nugraha
3
a
Post Graduate Student of Master of Immunology, Faculty of Pasca Sarjana, Universitas Airlangga,Indonesia
b
Department of Stem Cell Institute of Tropical Disease, Universitas Airlangga, Indonesia
2
Department of Microbiology Clinic, Faculty of Medicine, Dr. Soetomo Hospital, Indonesia
3
Department of Patology Clinic, Faculty of Medicine, Dr.Soetomo Hospital, Indonesia
Keywords: Structure Granuloma, TNF-α, Mycobacterium tuberculosis.
Abstract: Mycobacterium tuberculosis is a bacterium causes pulmonary tuberculosis, acid-resistant bacteria,
intracellular life and cause of death than other infectious diseases. The principle of immune response by the
formation of granulomas that prevent bacteria spread to other cells. TNF-α plays an important role in the
body's defense against intracellular bacterial infections and the invitro model maintaining granulomas and
the role of collagen in maintaining granulomas that prevent infection from becoming active. The purpose of
this study is to analyze the number of lymphocyte, monocytes/macrophages and levels of TNF-α between
collagen and without collagen. This study used healthy PBMC cells in mycobacterium tuberculosis bacterial
infection with MOI 1: 0.1 then divided into 2 groups the addition collagen and without collagen observation
in day 1, day 3, day 5, and day 7. HE stained was performed to calculate lymphocyte, monocyte/
macrophage and supernatant cells used to check TNF-α levels by ELISA method. The results showed no
effect of the addition of collagen to the number of lymphocytes, monocytes/macrophages and TNF-α levels.
Levels of TNF-α highest on day 3 and decresed on day 7.
1 INTRODUCTION
Tuberculosis (TB) is an infectious disease caused by
the bacterium Mycobacterium tuberculosis has
caused more deaths during the last 200 years
compared to other infectious diseases (Paulson,
2013). World Health Organization (WHO) data by
2015, an estimated 10.4 million new TB cases
worldwide. Six countries accounted for 60% of new
TB cases, including India, Indonesia, China, Nigeria,
Pakistan and South Africa (WHO, 2016).
Tuberculosis disease in addition to causing active
infection can also cause latent infections that are
asymptomatic conditions making it difficult for
treatment because it does not cause symptoms. In the
host body infected with Mycobacterium tuberculosis
mostly develops into a latent infection in comparison
with active infection at the host's primary defense
against Mycobacterium tuberculosis infection with
granuloma formation, ie the formation of organized
cell aggregates. (Fitzgerald et al., 2014).
Mycobacteria belong to the host by means of
aerosol will then be in the alveolar macrophages by
internalization by way of Mycobacterium
tuberculosis bacteria secrete virulence factors
namely ESAT-6 (Early Secreted Antigenic Target of
6 kDa), which in the secretion of through the system
the secretion of Mycobacterial type VII (ESX-1).
ESX-1 mediated translocation m.tuberculosis from
the macrophage cytoplasm into a phagolysosome is
the virulence of pathogenic Mycobacteria (Parasa et
al., 2014).
Mycobacterium tuberculosis bacteria are
internalized by macrophages releasing Interleukin 8
(IL-8) which is a strong chemoatractant for T
lymphocytes because it produces a bacterial antigen
so as to recruit T cells. T lymphocytes then become
active and produce gamma interferon (IFN-ɣ) which
serves to activate other macrophages , increasing the
production of Tumor Necrosis Factor Alpha (TNF-
α) and activating the metabolism of oxidative
macrophages and antimicrobial activity. TNF-α
plays a very important role in the development of
granulomas for cellular organization and
maintenance of granulomas, TNF-α mediates acute
phase responses that give rise to systemic symptoms,
356
Pengesti, I., Widodo, A. and Nugraha, J.
The Effect Collagen to Granuloma Structure annd Immune Response on Granuloma Tuberculosis Invitro Models.
DOI: 10.5220/0007542803560360
In Proceedings of the 2nd International Conference Postgraduate School (ICPS 2018), pages 356-360
ISBN: 978-989-758-348-3
Copyright
c
2018 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

and is necessary for the development and the
strength of granulomas that play an important role in
the accumulation and differentiation of macrophages
into epithelioid cells on mature granulomas
(Birkness et al., 2007 and Fitzgerald et al., 2014).
Granulomas are not always awake, bacteria
Mycobacterium tuberculosis can be reactivated.
During reactivation, granulomas become catalase
and then necrotic, and infected macrophages secrete
MMP-1, excessive secretion leads to collagen
degradation and tissue damage, causing M.
tuberculosis to enter the respiratory tract and causing
active infection. (Salgame, 2011)
The damage to collagen is an early onset of
immunopathology in tuberculosis, causing necrosis
and building an immune response, revealing the role
of extracellular matrix in regulating host and
pathogen interactions (Al Shammari et al., 2015)
Type I collagen is a fibril structure in the lung
and is highly resistant to enzymatic degradation. In
addition to biomechanical properties, type I collagen
has an important role in cell survival, adhesion,
proliferation, and migration (Brilha et al., 2017).
This study reports on the effect of collagen
administration on the structure of granuloma and the
secretion of Tumor Necrosis Factor (TNF) -α in
granuloma tuberculosis in vitro models.
2 MATERIALS AND METHODES
2.1 RPMI
Roswell Park Memorial Institute (RPMI) 1640.
RPMI 1640 media is a medium used for cell and
tissue culture, usually used for the growth of human
lymphoid cells. RPMI 1640 uses the bicarbonate
buffer system so that it enables the growth of several
types of cells, especially T lymphocytes,
hybridomas.
2.2 PBMCs
Peripheral Blood Mononuclear Cells = PBMCs are
cells made from human blood which are then
processed for the PBMC cell capture. The
concentrations of PBMC used in this study was 10
6
in each well.
2.3 Mycobacterium Tuberculosis
This study used bacterial isolates Mycobacterium
tuberculosis strain H37Rv with concentration 10
5
in
each well.
2.4 Extracellular Matrix
The extracellular matrix used collagen. The collagen
used has a number Cat # 04902 as a solution. In this
study, 950 μL of collagen was added with 50 μL
PBS 10 × and add 10uL NaOH 1N, then in PH
check, with neutral PH result.
3 RESULTS
3.1 Direct Granuloma Observation
The method used for direct observation is performed
directly under an inverted microscope using
specimens of living cell cultures in the plate / well.
Figure 1: Direct observation with infection (400x
magnification)
The figure 2 seen there is aggregate it indicates a
response to infection.
The Effect Collagen to Granuloma Structure annd Immune Response on Granuloma Tuberculosis Invitro Models
357
3.2 Calculation Count of Cells on the
Structure Granuloma
Figure 2: HE staining (400x magnification)
Figure 2 is the result of HE staining, the
measurement of lymphocyte cell distance and
monocyte / macrophage cells can be identified and
calculated on the granuloma structure.
Table 1. Average amount of cells constituent of granuloma
structure
Treatment Cells Day
1 3 5 7
With
Collagen
lymphocyte 14.5 11.33 5.17 2
monocyte /
macrophage
1.33 1.16 0.83 0.5
Without
Collagen
lymphocyte 10.5 9.83 3.83 2.333
monocyte /
macrophage
1.33 1 0.67 0.33
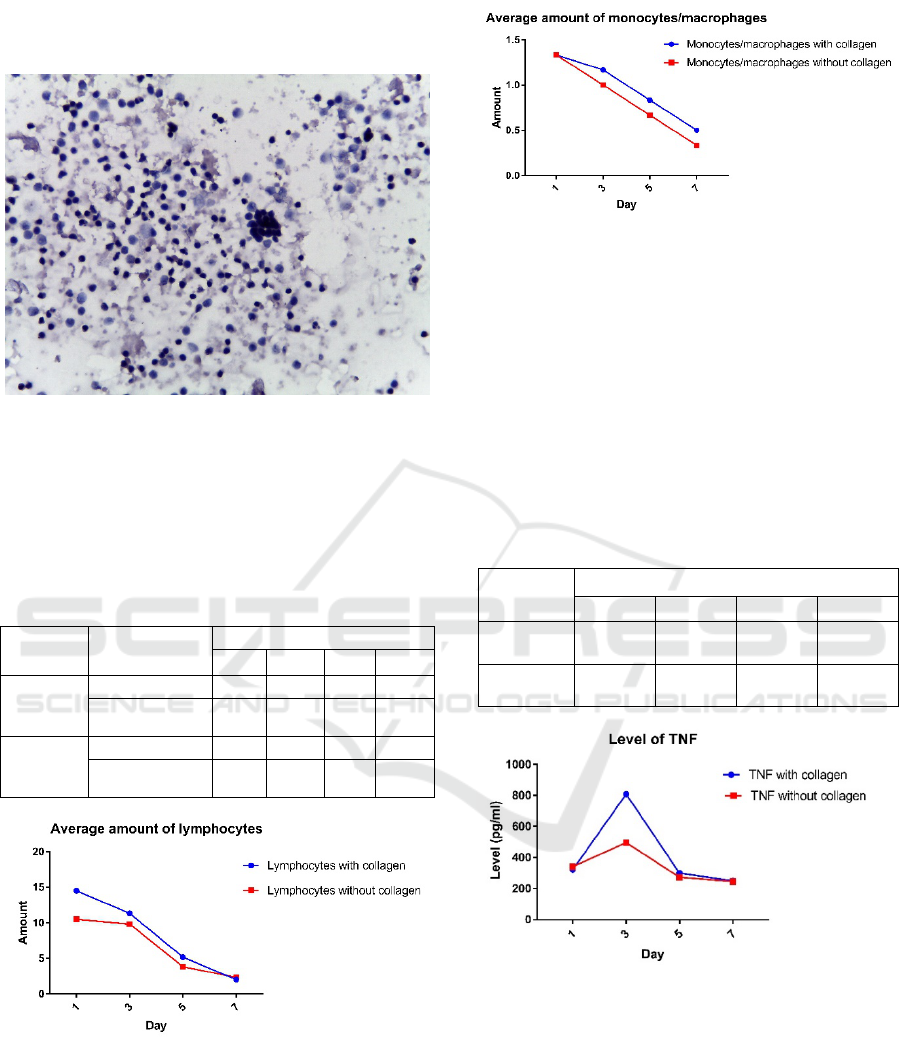
Figure 3: Average amount of lymphocytes
Figure 4:Average amount of monocytes/macrophages
Average number of cells of lymphocytes and
monocytes/macrophages shown in Figure 3 and
Figure 4. With paired t test P value of lymphocyte
cell count = 0.1662 and monocyte cell number the
value P = 0057 so there is no effect of collagen on
the amount lymphocyte and
monocytes/macrophages.
3.3 Examination the Levels of TNF-α
Table 2. Average levels of TNF-α
Treatment Day (pg/ml)
1 3 5 7
With
Colla
g
en 321.051 808.725 299.990 249.786
Without
Collagen 340.997 496.227 272.972 245.512
Figure 5: average levels of TNF-α based on variations day
With Paired t test P-value = 0.3744 or P > 0.05 so there is
no effect of collagen on the TNF-α.
3.4 Discussion
The formation of a granuloma is a dynamic process.
Formation of granuloma can be divided into three
phases, first phase is the stage where the innate
granuloma formed from macrophages and
neutrophils. The second phase immune granuloma
ICPS 2018 - 2nd International Conference Postgraduate School
358

formed after the emergence of specific antigen of T
cells. The third phase chronic granuloma comes
from the difference in the morphology and the
change of the structure of the granuloma. After the
M. Tb root is infected with Alveolar Macrophages
(MA) then it goes earlier against the inflammatory
response. In the meantime, it strengthens the
immune response of the host then the recruitment of
innate immune cells against the new target M. Tb
and contribute to spreading M.Tb. The
mycobacterium species is inhibited by the fusion
process fagolysosome. It is associated with
virulence, the strain of relativity M.Tb inhibits
fusion. Infected macrophages produce a number of
pro-inflammatory cytokines and chemoatractant
cytokines TNF-α, IL-6 and IL-8, which facilitate the
recruitment of macrophages and granulocytes into
new infections and lead to the formation of
congenital granulomas (Shaler et al., 2013).
The chronic Granuloma phase causes significant
changes in morphology and granuloma function. In
infected individuals a spectrum of granuloma
structures, the classification of either bacterial or
non-bacterial lesions and fibrotic necrotic
granulomas, suggests that granuloma evolution is a
highly dynamic process (Shaler et al., 2013).
The pathologic infection of tuberculosis in
humans is an organized aggregate granuloma that is
organized from immune cells consisting of
macrophages, lymphocytes and immune cells
present in the host (Cadena A.M, et al, 2017).
Formation of granuloma phase of immune
granuloma will produce continuous chemokine by
APC in infected lung and efficiently recruit T cells.
Then, T cells will surround and close the infected
macrophages by M.Tb bacteria. T cell activation
serves as bactericide and limits bacterial mobility
thus it prevents the spread of bacteria to other cells.
The arrival of T cells and the formation of immune
granulomas are associated with the growth of stable
bacteria (Mogues et al., 2001).
Monocyte / macrophage cells will clump in
response to Mtb infection and form a structure such
as granuloma and initiate granuloma formation. It is
defined as a grouping of monocytes / macrophages
during inflammation, an initial occurrence during
mycobacterial infection (Parasa et al, 2014).
Macrophages will secrete IL-8 as a strong
chemoatractant for T lymphocytes that will surround
the granuloma structure. T lymphocytes then secrete
IFN-ɣ to activate additional macrophages.
Macrophages will produce TNF-α and play an
important role in the accumulation of macrophages
and other cells (Fitzgerald et al., 2014).
Tumor Necrosis Factor Alpha (TNF-α) is a
cytokine that emerges since early inflammation
plays an important role in the mechanism of the
innate immune response. TNF-α is an autocrine
cytokine produced by macrophages, dendritic cells,
lymphocytes, neutrophils, mast cells, and endothelial
cells and performs functions such as chemotaxis
with the formation of granulomas (Sasindran and
Torrelles, 2011).
4 CONCLUSIONS
The invitro is a risky method, since the
contamination is likely to occur. Therefore, it is
suggested to absolutely ensure the sterile condition
before conducting the isolation, the tools, materials,
specimens.
REFERENCES
Al Shammari, B., Shiomi, T., Tezera, L., Bielecka, M. K.,
Workman, V., Sathyamoorthy, T., … Elkington, P. T.
(2015). The Extracellular Matrix Regulates
Granuloma Necrosis in Tuberculosis. Journal of
Infectious Diseases, 212(3), 463–473.
https://doi.org/10.1093/infdis/jiv076
Birkness, K. a, Guarner, J., Sable, S. B., Tripp, R. a,
Kellar, K. L., Bartlett, J., & Quinn, F. D. (2007). An in
vitro model of the leukocyte interactions associated
with granuloma formation in Mycobacterium
tuberculosis infection. Immunology and Cell Biology,
85(2), 160–168. https://doi.org/10.1038/sj.icb.7100019
Brilha, S., Wysoczanski, R., Whittington, A. M.,
Friedland, J. S., & Porter, J. C. (2017). tuberculosis
Infection, 1(10).
https://doi.org/10.4049/jimmunol.1700128
Cadena, A. M.,Sarah M. Fortune and JoAnne L.Flynn
(2017). Heterogeneity in tuberculosis.
doi:10.1038/nri.2017.69
Davis,J.M.,and Ramakrishnan,L. (2009). The role of the
granuloma in expansion and dissemination of early
tuberculousis infection. Cell .136,37–49
Fitzgerald, L. E., Abendaño, N., Juste, R. A., & Alonso-
hearn, M. (2014). Three-Dimensional In Vitro Models
of Granuloma to and Resuscitation of Dormant
Mycobacteria, 2014, 8
Kapoor, N., Pawar, S., Sirakova, T. D., Deb, C., Warren,
W. L., & Kolattukudy, P. E. (2013). Human
Granuloma In Vitro Model, for TB Dormancy and
Resuscitation. PLoS ONE,8(1).
https://doi.org/10.1371/journal.pone.0053657
Mogues,T.,Goodrich,M.E.,Ryan, L., Lacourse,R., and
North,R. J.(2001).The relative importance of T cell
subsets in immunity and immunopathology of air
The Effect Collagen to Granuloma Structure annd Immune Response on Granuloma Tuberculosis Invitro Models
359

borne Mycobacterium tuberculosis infection in mice.
J. Exp.Med. 193, 271–280.
Parasa, V. R., Rahman, M. J., Ngyuen Hoang, A. T.,
Svensson, M., Brighenti, S., & Lerm, M. (2014).
Modeling Mycobacterium tuberculosis early
granuloma formation in experimental human lung
tissue. Disease Models & Mechanisms, 7(2), 281–288.
https://doi.org/10.1242/dmm.013854
Paulson T. Epidemiology: A mortal foe. (2013).
Nature.;502(7470):S2–S3.
Salgame, P. (2011). MMPs in tuberculosis: Granuloma
creators and tissue destroyers. Journal of Clinical
Investigation, 121(5), 1686–1688.
https://doi.org/10.1172/JCI57423
Sasindran, S. J and Torrelles J. B. (2011). Mycobacterium
tuberculosis infection and inflammation: what is
beneficial for the host and for the bacterium?. Cellular
and Infection Microbiology, 2(2).
doi:10.3389/fmicb.2011.00002
Shaler, C.R., Carly N.Horvath, Mangalakumari
Jeyanathan and Zhou Xing (2013). Within the
Enemy’s Camp: contribution of the granuloma to the
dissemination, persistence and transmission of
Mycobacterium tuberculosis. Frontiersin
Immunology,4(30).doi:10.3389/fimmu.2013.00030
Taylor,J.L.,Hattle,J.M.,Dreitz, S. A.,
Troudt,J.M.,Izzo,L.S., Basaraba,R.J., etal. (2006).Role
for matrix metalloproteinase 9 in granuloma formation
during pul monary Mycobacterium tuberculosis
infection. Infect.Immun. 74, 6135–6144
WHO. (2016). Global Tuberculosis Report 2016. Cdc
2016, (Global TB Report 2016), 214.
https://doi.org/ISBN 978 92 4 156539 4
ICPS 2018 - 2nd International Conference Postgraduate School
360
