
The Potential of Protein Ghrelin as Material for Energy Balance
Setting for Feed Efficency in Broiler Chicken
Nove Hidajati
1
, Anwar Ma’ruf
2
and Ratna Damayanti
1
1
Department Basic of Veterinary Medicine, Faculty Veterinary Medicine, Universitas Airlangga, Surabaya,
2
Postgraduate School, Universitas Airlangga, Surabaya
Keywords: Ghrelin, energy, feed efficiency, broiler.
Abstract: The purpose of this study was to determine the molecular weight of the protein ghrelin as a basis to
determine amino acid composition of protein ghrelin and subsequently to make synthetic ghrelin protein
whose function is to control energy balance in broilers. Samples were isolated from the digestive tract and
brain tissue of the broilers and then examined by SDS Page and Western blot. Based on the results, it can
be concluded that the protein ghrelin had the molecular weight of 44 kDa and neuropeptide Y of 11 kDa.
1 INTRODUCTION
Ghrelin and leptin are complementary but work
antagonistically. Their signals reflect acute or
chronic energy balance changes and their effects are
mediated by hypothalamic neuropeptides such as
neuropeptide Y (NPY) and augouti related peptide
(AgRP) (Inui et al., 2004).
Gastric distension and gastric
hyposensitisation are insufficient to stimulate ghrelin
response. This possibility is a postgastric process
involving insulin secretion, either directly or
indirectly, through the incretin stimulation of the
hormone glucagon such as peptide 1 and gastric
inhibitory peptide. Most studies suggest that insulin
will lower ghrelin concentrations that are
independent of glucose. The insulin mechanism
inhibiting the effect of ghrelin concentration is not
fully known. These insulin effects may be mediated
by the direct effects of ghrelin secreting cells or the
effects of humoral mechanisms or central
mechanisms (Bloom, 2005).
Association between ghrelin, stomach,
hypothalamus and the implications of ghrelin on
gastrointestinal function control, energy balance, and
current growth has not been entirely clear.
Therefore, a study is needed to find ghrelin amino
acid from broiler chickens so that we can create
synthetic
ghrelin protein that can be used to regulate the
energy balance and growth of the livestock.
2 MATERIALS AND METHODS
This study used samples of male Lohman (MB 202
P) broiler chickens which were maintained from the
age of 1 day up to 21 days in letter cages, as many as
25 chicken.
Day old chicken were placed in a letter
cage for 21 days with food and drink ad libitum.
After reaching the age of 21 days, the chickens were
sacrificed to be sampled in the form of
gastrointestinal and brain tissue for the following
tests (1) Isolation of ghrelin and neuropeptide Y
(NPY) proteins from the gastrointestinal tract and
brains, (2) Identification of ghrelin and neuropeptide
proteins (NPY) of the gastrointestinal tract and
brains of broilers using SDS-PAGE (sodium dudecyl
sulphate polyacrylamide gel electrophoreses)
method, (3) Analysis of molecular weight of ghrelin
protein and Neuropeptide Y by blotting method i.e.
Western Blot technique using proteins described
electrophoresis of polyacrylamide gel, (4)
Examination of amino acid structure of ghrelin and
neuropeptide Y by MALDI-TOP method.
528
Hidayati, N., Ma’ruf, A. and Damayanti, R.
The Potential of Protein Ghrelin as Material for Eenergy Balance Setting for Feed Efficency in Broiler Chicken.
DOI: 10.5220/0007546305280531
In Proceedings of the 2nd International Conference Postgraduate School (ICPS 2018), pages 528-531
ISBN: 978-989-758-348-3
Copyright
c
2018 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
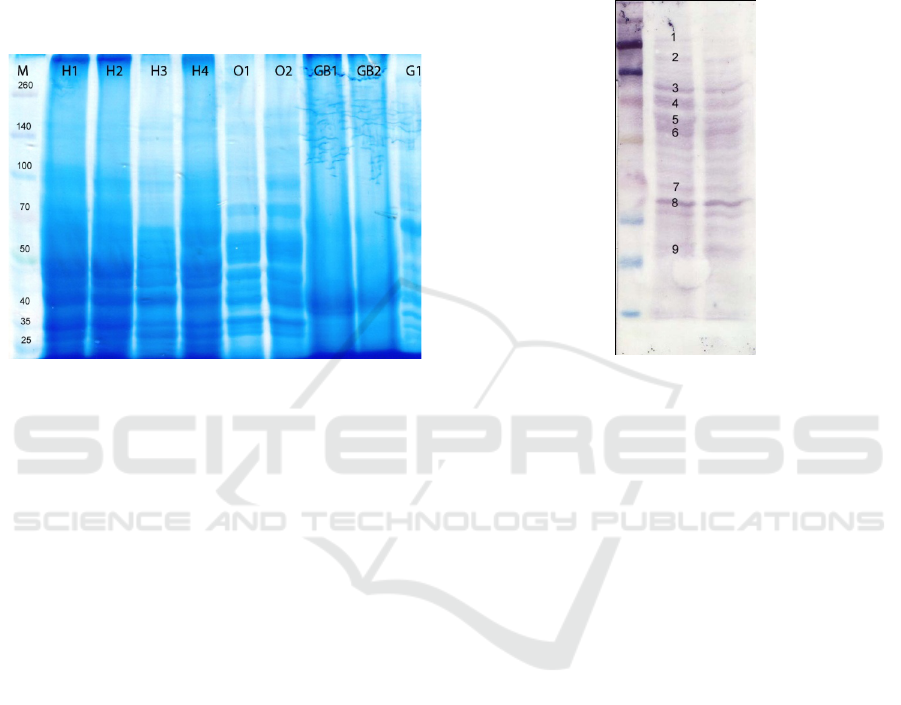
3 RESULTS AND DISCUSION
3.1 SDS Page for Ghrelin and
Neuropeptide Y Proteins
Results SDS-PAGE ghrelin and neuropeptide Y
(NPY) proteins in the broiler's gastrointestinal tract
and brain showed the presence of ghrelin and
neuropeptide Y proteins, as shown in Figure 1.
Figure 1 : SDS page of ghrelin and NPY proteins from the
digestive tract and brain.
The results of SDS-PAGE on gastrointestinal
tract and brains of broilers revealed ghrelin protein
and neuropeptide Y. SDS-PAGE results showed that
there were several visible bands. One protein band
was found each in the markers between 260 and 140
kDa, 140 and 100 kDa, 100 kDa and 50 kDa, 50 kDa
and 40 kDa, and between 25 kDa and 10 kDa.
Protein bands formed between 50 kDa and 40
kDa markers and between 25 kDa and 10 kDa
markers were suspected as ghrelin and neuropeptide
Y proteins. The protein bands formed on the
gastrointestinal tract and the broiler's brain were
very clear, indicating that the tissue appears to
induce the strongest antibody antigen reaction.
SDS-PAGE protein of gastrointestinal tract and
brain of broiler chicken showed protein band
between 50 kDa and 40 kDa markers, which were
protein with molecular weights of 44 kDa and 11
kDa, but it has not been certain whether it was
ghrelin protein and neuropeptide Y as several other
protein bands were also formed between these
markers. To prove that the formation of protein
bands with molecular weight of 44 kDa and 11 kDa
was ghrelin and neuropeptide Y protein, it was
necessary to perform further examination.
3.2
Western Blot for Protein Ghrelin
from Broiler’s Digestive Tract
The Western blot of ghrelin protein in
gastrointestinal tissue showed the presence of a 44
kDa molecular weight of ghrelin protein, as diplayed
in Fig. 2.
Figure 2 : Western blot for ghrelin protein from broilers’
digestive tract
The result of ghrelin protein molecular weight
calculation showed that the molecular weight of
ghrelin protein was 44 kDa. The formation of
protein bands between 50 kDa and 40 kDa markers,
after being calculated, apparently showed a molecule
with molecular weight of 44 kDa. This suggested
that the protein produced by SDS-PAGE tested with
Western blot was a ghrelin protein of growing-phase
broiler with a molecular weight of 44 kDa. The
formation of the protein band with 44 kDa molecular
weight was definite because there was a binding
between protein ghrelin resulted from SDS-PAGE
and rabbit pAb ghrelin (data Sheet Rev. 102203F).
3.3
Western Blot for NPY Protein from
Broiler’s Brain
The result of Western blot protein of neuropeptide Y
on brain tissue showed the existence of Y
neopopeptide protein with 11 kDa molecular weight,
as shown in Figure 3.
The Potential of Protein Ghrelin as Material for Eenergy Balance Setting for Feed Efficency in Broiler Chicken
529
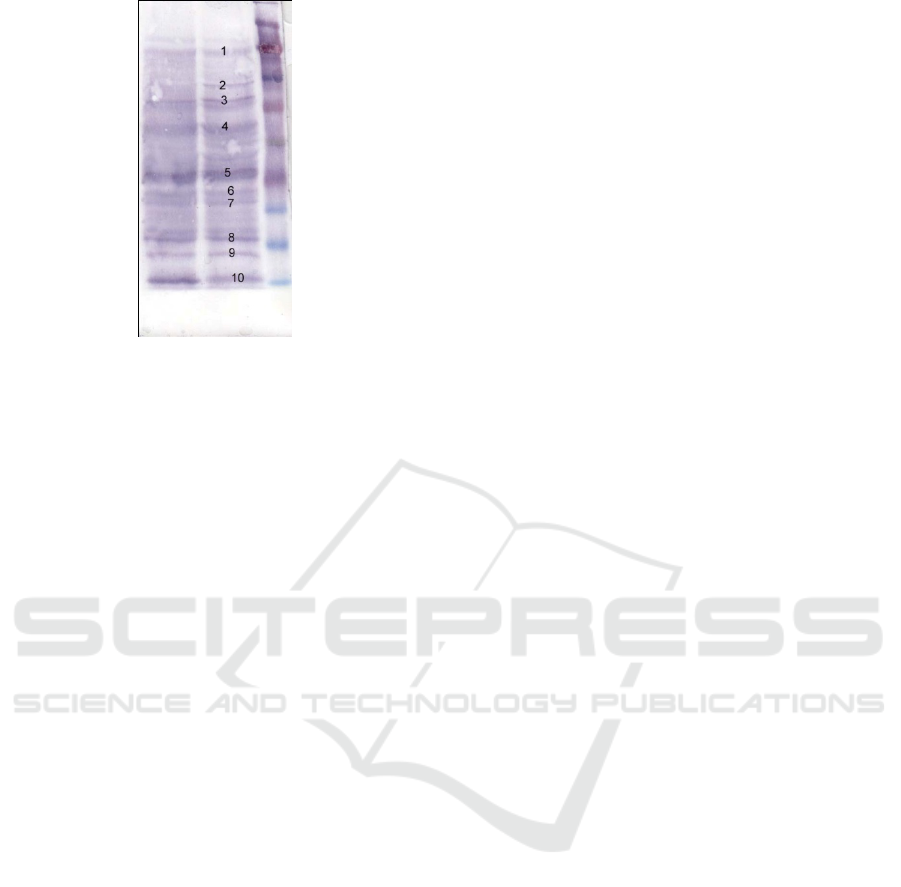
Figure 3 : Western blot for neuropeptide Y protein from
broiler’s brain.
The results of the molecular weight calculation
of neuropeptide Y protein showed that it had a
molecular weight of 11 kDa. The formation of
protein bands between 25 kDa and 10 kDa markers,
after being calculated, was found to be 11 kDa. This
suggested that the SDS-PAGE protein tested with
Western blot was a neuropeptide Y protein of
growing phase broiler chicken with a molecular
weight of 11 kDa. The formation of a protein band
of 11 kDa molecular weight was definite because
there was a binding between the protein ghrelin
resulting from SDS-PAGE with neupeptide Y
antibody (data Sheet ab30914).
Ghrelin is a gastric peptide that plays an
important role in the regulation of food into the body
(food intake). Before eating the plasma, ghrelin
concentration rises gradually and immediately goes
down after eating. The addition of ghrelin
intravenously increases food intake and appetite,
which proves that ghrelin plays a role in hunger and
the beginning of a meal initiation. Ghrelin is also
involved in weight control because the body mass
index is negatively controlled by plasma ghrelin
concentrations at the time of fasting. Abnormalities
of the signal from the stomach signal is related to
energy balance disorders and growth, and this is
related to gastrointestinal and neuroendockrine
function.
Ghrelin and leptin are complementary but work
antagonistically, their signals reflect acute or chronic
energy balance changes and their effects are
mediated by hypothalamic neuropeptides such as
neuropeptide Y (NPY) and augouti related peptide
(AgRP).
4 CONCLUSIONS
The molecular weight of ghrelin protein was 44 kDa
with amino acid structure consisted of methionine,
phenylalanine, leucine, arginine, valine, isoleucine,
leucine and neuropeptide Y molecular weight was
11 kDa with threonine, methionine, arginine,
leucine, tryptophan, valine, serine, valine, leucine,
threonine, leucine, alanine, glutamate, alanine,
tyrosine, proline, and serine. By identifying the
molecular weight and the arrangement of amino
acids, we can create synthetic ghrelin protein to
regulate the energy balance of broiler chickens.
REFERENCES
Cummings DE, Purnell JQ. Frayo RS, Schmidova
K.Wisse BE and Weigle DS. Apreprandial
rise in plasma ghrelin level suggests a role in
meal initiation in humans. Diabetes. 2001.50.
1714-1719.
Haqq AM, Farooqi IS, O’Rahilly S, Stadler DD,
Rosenfeld RG, Pratt KL, LaFranchi SH,
Purnell JQ. 2003. Serum ghrelin levels are
inversely correlated with body mass index,
age, and insulin concentrations in normal
children and are markedly increased in
Prader-Willi syndrome. J Clin Endocrinol
Metab 88:174–178.
Hiejima H, Nishi Y, Hosoda H. 2009. Regional
distribution and the dynamics of n-decanoyl
ghrelin, another acyl-form of ghrelin, upon
fasting in rodents. Regulatory Peptides, vol.
156, no 1-3, pp 47-56.
Kaiya Hiroyuki, Saito Ei-Suke, Tachibana Tetsuya,
Furuse Mitsuhiro, Kangawa Kenji. 2007.
Changes in ghrelin levels of plasma and
proventriculus and ghrelin mRNA of
proventriculus in fasted and refed layer
chicks. Domestic Animal Endocrinology.
Elsevier. 32 (2007) 247-259.
Kojima M, Hosoda H & Kangawa K.2004. Clinical
endocrinology and metabolism. Ghrelin, a
novel growth-hormone-releasing and
appetite-stimulating peptide from stomach.
ICPS 2018 - 2nd International Conference Postgraduate School
530

Best Pract Res Clin Endocrinol Metab 18,
517–530.
Saito E, Kaiya H, Takagi T, Yamasaki I, Denbow
DM, Kanagawa K, and Furuse
M.2002.Chicken ghrelin and growth
hormone-releasing peptide-2 inhibit feed
intake of neonatal chicks. Eur. J. Pharmacol.
453:75-79.
Saito E, Kaiya H, Tachibana T, Tomonaga S, DM
Denbow DM, Kanagawa K and Furuse
M.2005. Inhibitory effect of ghrelin on food
intake is mediated by the corticotrophin
releasing factor system in neonatal chicks.
Reg. Pept. 125: 201-208.
Wang HJ, Geller F, Dempfle A, Schauble N, Friedel
S, Litchner P, fontenla-Horro F, Wudy S,
Hagemann S, Gortner L, Huse K,
Remschmidt H, Bettecken T, Meitinger T,
Schafer H, Hebebrand J and Hinney A,
Ghrelin receptor gene: identification of
several sequence variants in extremely obese
children and adolescents, healthy normal-
weight and underweight students, and
children with short normal stature. Journal of
Clinical Endocrinology and Metabolism
2004.89.157-162.
The Potential of Protein Ghrelin as Material for Eenergy Balance Setting for Feed Efficency in Broiler Chicken
531
