
The Impact of Limescale on Home Appliances in a Building
Hajji Abdelghani
1
, Abbou Ahmed
1
and El Boukili Abdellah
2
1
Mohammedia School of Engineering, EMI, Ibn Sina Avenue,Mohamed V University, Agdal, Rabat, Morocco
2
Higher Education School, ENS, Mohamed V University, P4014, Takadoum, Rabat, Morocco
Keywords: Hard Water, Limescale, Hydraulic Devices, Experience, Matlab, Softener.
Abstract: The problem of limescale in hydraulic devices that produce or use hot water is a well-known phenomenon in
everyday life. It is common in the following appliances: electric water heater, washing machine, dishwasher,
coffee maker, electric kettle, etc.
To overcome this problem, several approaches exist and can be applied to the different levels of the hot water
production system.
This work enables to characterize the drinking water of some regions in Morocco, looks for conditions that
minimize limescale production and shows experimentally that this latter has a significant effect on the energy
bill of a building.
The results show that it is preferable to introduce a water filtering system (softener), especially in areas where
water is hard or very hard. This will be applied to the building's water supply to reduce the energy bill, extend
the life of hydraulic installations, reduce the frequency of maintenance, make soap and detergents more
efficient and also improve the quality of drinking water.
1. INTRODUCTION
The energy efficiency of the building is a hot topic. In
Morocco, a lot of researchers are interested in this
topic because the building is the biggest energy
consumer, before the transport and the industry. It
also represents 25% of national carbon dioxide
emissions. [Abarkan, 2014]
It is considered today as the fourth energy after
fossil fuels, renewable energies and nuclear energy.
The ambition of the Kingdom of Morocco is to ensure
a better use of energy in all areas of economic and
social activity, considering the need to rationalize and
improve the consumption of energy to meet the
growing energy needs of our country. [Law 47-09 on
Energy efficiency 2015]
Buying economical household appliances is not
sufficient since much of the electrical consumption of
a piece of equipment depends on how it is used and
how it is maintained throughout its life. [
ADEREE,
(n.d.)
]
The phenomenon of limescale formation occurs in
cold water urban distribution systems and more
intensively in the heat transport circuits of industrial
plants and in hydraulic devices that produce or use hot
water.
The technological and economic consequences of
scaling are varied:
Loss of efficiency due to the insulating
power of limescale, which increases the
energy consumption (10 mm of limestone on
the electrical resistance can increase losses
up to 50%). [ASPEC SERVIGAZ, (n.d.)]
Shortening of the life length of the already
expensive equipment.
Rise in the temperature of the appliances
with the risk of destruction by overheating.
The malfunction of the hydraulic devices.
A progressive reduction of the pipes sections
with an increase of pressure losses or even
their obstruction.
Abdelghani, H., Ahmed, A. and Abdellah, E.
The Impact of Limescale on Home Appliances in a Building.
DOI: 10.5220/0009773804230428
In Proceedings of the 1st International Conference of Computer Science and Renewable Energies (ICCSRE 2018), pages 423-428
ISBN: 978-989-758-431-2
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
423

In addition, tartar in large quantities is an
agent promoting the development of certain
bacteria such as Legionella. [
Hadfi A., 2012]
Our research work, conducted by the
Mohammedia School of Engineering (Mohamed V
University of Rabat), begins with the measurement of
the hardness of drinking water in 4 regions in
Morocco. Then we will try to find, theoretically, the
conditions that minimize the quantity of limescale in
hot water taking into account the comfort and health
of the occupants of the building.
Subsequently, we will show, experimentally, that
drinking water which contains the higher quantity of
limescale (higher TH) will require more energy to
heat.
Finally, we will lead a comparative study about
the energy consumption of the various hydraulic
devices of a building using different waters.
2. RELATED WORK
There are very few published research data on the
energy impact of limestone on home appliances.
Lerato Lethea (2017) has studied the impact of
water hardness on the energy consumption of geyser
heating elements. That study proved that the scale
formation of 1.5 kW and 3 kW geyser heating
elements because of high total water hardness rised
the energy consumption by about 4% to 12%. It
proposed an energy efficient electronic descaling
technology. In my opinion, it is a good thing but it is
necessary to act before the scale is left in large
quantities. We suggest, therefore a softener which
slows down scaling.
On the other hand, Konstadinos Abeliotis (2015)
studied the impact of water hardness on consumers’
perception of laundry in five European countries.
He showed that the hardness of water is a key
factor in the success of the washing process project.
For the first time, a research was conducted in five
European countries aimed at identifying consumers'
perceptions about the effect of water hardness in
washing performances. In terms of water hardness,
the respondents seem to be well aware that the areas
in which they live, face problems due to the hardness
of water. The results also indicate that satisfaction
with the washing result, although related to high
levels, depends on the hardness of water.
In the same study, we observe that the use of
softened drinking water in households has several
positive effects, such as the reduction in energy
consumption.
In the same context, Bruce A. Cameron (2011)
worked on consumers’ detergent considerations: hard
water laundering - How much additional detergent is
needed?
He showed that liquid detergents wash in both
fresh and hard water. Powdered detergents were more
efficient than liquids in fresh water. The hardness of
water affected powdered detergents and, depending
on the type of detergent, 10-15% to over 30%
additional detergent was needed to achieve a similar
result to that of fresh water.
The last two studies got interested in the effect of
water hardness in the washing machine and they tried
to study the effect of all appliances in a building that
use hot water.
3. WATER HARDNESS
MEASUREMENT
We will begin this work by measuring experimentally
the hardness of drinking water in four regions of
Morocco. The hardness, called the hydrotimetric title
(TH), corresponds to the totality of the calcium and
magnesium salts:
TH = [Ca
2+
] + [Mg
2+
] (1)
3.1. Equipment
The equipment that has been used in this study is the
material that allows the experimental determination
of the TH hardness of water:
drop sensor - LabQuest interface – eriochrome black
T (NET) - tetraacetic ethylene diamine (EDTA) -
buffer solution 5 ml (milliliter) - erlenmeyer 250 ml -
magnetic stirrer and stir bar.
3.2. Method
The method to determine the total hardness of water
is based on complexation assays to form very stable
complexes between a central ion (Calcium,
Magnesium) and an EDTA ligand.
In a 250-millilitre-Erlenmeyer flask, V
water
= 50 ml
of drinking water to be analyzed is added. 5 ml of the
buffer solution and one drop of the NET indicator are
added and then, the mixture is titrated with EDTA
solution. The shift is reached when we get the royal
blue color.
The equivalence relation is written as:
ICCSRE 2018 - International Conference of Computer Science and Renewable Energies
424

[EDTA].V
eq
= ([Ca
2+
] + [Mg
2+
]).V
water
(2)
V
eq
: Volume of equivalence
It is shown that the TH in French degree unit,
noted ° F, is written as:
TH = 5.0,8.V
e
q
(ml) (3)
3.3. Results
Here are the results obtained for the samples from 4
regions in Morocco:
Table 1: TH values of 4 regions in Morocco.
Water sample E1 E2 E3 E4
TH in F ° 41,60 28,48 28,00 10,24
Nature of water very hard hard Hard soft
3.4. Discussion
Depending on where you live and on the soil geology
of your area, the water coming out of your faucet can
be more or less hard. This is why it is important to be
well-informed on this subject, otherwise you will deal
with a lot of inconveniences caused by the limescale
at home. [Union française des professionnels du
traitement de l’eau, (n.d.)]
Limescale is naturally present in water. Its
presence in small or large quantities depends on the
nature of the terrain crossed.
In table 1, it can be seen that water E1 is the
hardest one. It contains the higher quantity of
limescale compared to the waters of the other regions.
And water E4 is the softest one.
Hard water causes scaling of distribution
networks and soap excessive consumption; fresh
water can cause pipes’ corrosion. So, water hardness
should be moderated to ensure an acceptable balance
between corrosion and scaling. [Sante Canada, 1979]
4. STUDY OF THE EFFECT OF
TEMPERATURE AND PH ON
LIMESCALE FORMATION
We will study, theoretically, limescale dissolution
according to the following parameters: temperature T
and pH.
4.1. Material
In this study we will use the Matlab software.
4.2. Method
To study limescale dissolution at temperature T and
pressure p, it is assumed that:
Limescale is assimilated to calcium
carbonate CaCO
3
(s).
The liquid phase is in equilibrium with the
gas phase with respect to carbon dioxide
exchanges.
The ions activities are almost equal to the
ions molar concentrations. [Cortial, N,
(n.d.)]
The following reactions come into play:
CaCO
3
(s) = Ca
2+
+ CO
3
2-
(K
S
: solubility product)
CO
2
+ 2H
2
O = HCO
-
+ H
3
O
+
(K
a1
:acidity constant 1)
HCO
-
+ H
2
O = CO
3
2-
+ H
3
O
+
(K
a2
: acidity constant 2)
The solubility S of calcium carbonate is defined by
[Cortial, N, (n.d.)]:
S=[CO
2
]+[HCO
-
]+[CO
3
2-
] (4)
We can show that:
S=(10
X-2.pH
+ 10
Y-
p
H
+10
-Z
)
0.5
(5)
With: X =
pK
a1
+ pK
a2
- pK
S
Y =
pK
a2
- pK
S
Z =
pK
S
The Impact of Limescale on Home Appliances in a Building
425
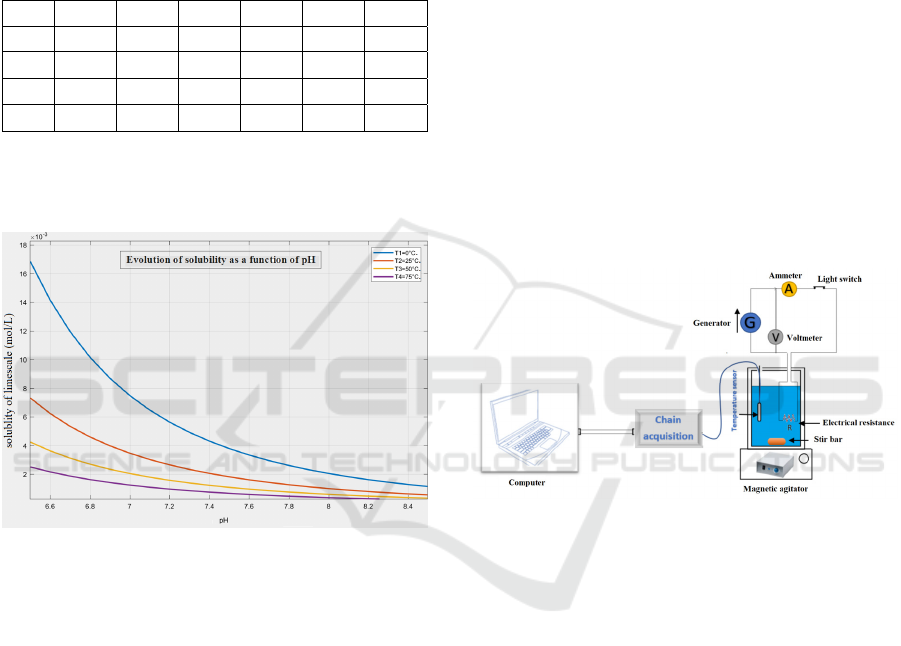
4.3. Results
Here are the numerical values of the parameters for
different temperatures [Cortial, N, (n.d.)]:
Table 2: Values of parameters.
T
(°C) pK
a1
pK
a2
pK
S
X Y Z
0 6,583 10,63 8,022 9,191 2,608 8,022
25 6,368 10,33 8,341 8,357 1,989 8,341
50 6,296 10,17 8,625 7,841 1,545 8,625
75 6,186 9,99 8,862 7,314 1,128 8,862
Here is the representation of the solubility as a
function of pH for different temperatures under the
Matlab environment:
Figure 1: Solubility of limescale as a function of pH for
different temperatures.
4.4. Discussion
Temperature has a significant influence on the
solubility of calcium carbonate. The latter increases
the presence of carbon dioxide. Indeed the increase in
temperature decreases the amount of dissolved
carbon dioxide and causes the precipitation of
calcium carbonate. [Hadfi A., 2012]
From figure 1, we notice that the pH rise favors
the formation of limescale. And the increase in
temperature favors the precipitation of calcium
carbonate. To minimize the quantity of the formed
limescale and ensure comfort to the occupants, you
should thus adjust your appliances to moderated
temperatures between 55 and 60 ° C and the water pH
should be between 6,5 and 7. [Health misitry, 2006]
5. EVOLUTION OF THE ENERGY
SUPPLIED TO WATER
ACCORDING TO
TEMPERATURE
We will study the evolution of the energy supplied to
water as a function of temperature for different TH
values.
5.1. Equipements
Here is the material that makes this study possible:
calorimeter - temperature sensor - LabQuest chain
acquisition - computer - resistor 3Ω - 4 drinking water
samples - graduated cylinder - 6V voltage generator -
multimeter - magnetic stirrer and stir bar - connection
wires.
5.2. Method
Here is the experimental setup:
Figure 2: Experimental device.
The energy supplied to the water is calculated
using the following relation:
E = R.I
2
.Δt (6)
R: electrical resistance
I: current intensity (A)
Δt: required duration
We redo the experiment for the other samples.
ICCSRE 2018 - International Conference of Computer Science and Renewable Energies
426

5.3. Results
Here are the obtained experimental results:
Figure 3: Evolution of energy supplied to water as a
function of temperature T.
5.4. Discussion
In figure 3, the curves do not evolve in the same way
because of the water hardness. Indeed, the harder the
water, the more energy is required to get to the
apparatus’ temperature of use.
Consequently, drinking water E4 (harder) requires
more energy to heat water at the temperature of use of
the device.
6. COMPARISON OF ENERGY
CONSUMPTION IN THE
BUILDING IN TWO CASES
In this comparative study, we will estimate the annual
energy consumed by the hydraulic apparatus of our
building in the 2 extreme cases: water E4 of hardness
TH4 and water E1 of hardness TH1.
6.1. Devices
It is about some domestic appliances of a four-person-
house: dishwasher; washing machine; electric kettle;
electric water heater; coffee maker.
6.2. Method
The energy required to heat a volume V of water from
the temperature T
1
to the temperature T
2
per cycle of
each apparatus is calculated using the following
relation:
E
c
y
cle
= R.I
2
.Δt.V/V
0
(7)
V: Volume of water used by the device during a
cycle
V
0
: Volume of water used during the experiment
Annual energy is deducted for each device by
inducing the frequency of use:
E
annual
= 365.f.R.I
2
.Δt.V/V
0
(8)
f: frequency of use of the device per day
6.3. Results
In the table below, we can read the appliances of a
four-person-house.
Table 3: Appliances’ annual consumption.
Charact.
Appliances
Volume of
water/cycle
Operating T
(°C)
Frequency
of use
(D
- 1
)*
Consumed annual
energy in MJ
Water
E4
Water
E1
Dishwasher
20L 50 1 11,33 19,33
Washing
machine
50L 40 0,5 9,16 14,16
Electric
kettle
1L 100 4 12 18,93
Coffee
maker
0,5L 100 1 1,50 2,36
Electric
water heater
80L 60 2 133,33 218,66
Total annual energy for E4: 46,38 kWh
Total annual energy for E1: 75,85 kWh
* average values derived from devices catalogs
6.4. Discussion
It is confirmed that the annual consumption for hard
water is higher.
The relative difference between the two previous
energies is written as: ΔE/E = 38,85 %
More than 38% of the energy consumption of a
building's hydraulic equipment can be reduced if E4
water is used instead of E1 water.
We note that with fresh water we consume less
energy, thus it reduces the electricity bill of our
building.
The reduction of limescale in water also extends
the life length of our devices and reduces the
frequency of maintenance of these devices.
The Impact of Limescale on Home Appliances in a Building
427

7. CONCLUSION
The results show that it is preferable to introduce a
water filtering system (softener), especially in areas
where water is hard or very hard. This will be applied
to the building's water supply to reduce the energy
bill, extend the life length of hydraulic installations,
reduce the frequency of maintenance, make soap and
detergents more efficient and also improve the quality
of drinking water.
To ensure energy efficiency of buildings, it is
interesting to:
know the TH of drinking water used in the
building;
know the effects of parameters that favor the
formation of limescale;
choose the class of devices used in the
buildings;
properly use and adjust devices;
install a softener, etc.
PERSPECTIVES:
Studying the profitability of a softener in the
same building.
Analyzing the consequences of the
replacement of an electric water heater by a
solar one.
REFERENCES
Abarkan M., 2014, « Modélisation et Analyse du
comportement d’un Bâtiment équipé d’un Système
Multi Sources d’´énergie », PhD Thesis, Aix
University, Marseille and Sidi Mohamed Ben Abdellah
University, Fes, Morocco.
Law 47-09 on Energy efficiency, 2015. Available at:
http://www.aust.ma/images/aust/reglementation/Dahir
s/Dahir%201-11-161.pdf. Accessed: March 2018.
ADEREE, (n.d.) « Les bonnes pratiques de l’efficacité
énergétique des bâtiments ». Available at:
http://docplayer.fr/14141959-Les-bonnes-pratiques-
de-l-efficacite-energetique-dans-le-batiment.html.
Accessed: April 2018.
ASPEC SERVIGAZ, (n.d.), « EMBOUAGE:
Causes, Effets, et Résolutions de Problèmes ».
Available at: https://www.aspec-
servigaz.fr/index.php/desembouage/causes-effets-et-
resolution-de-lembouage. Accessed: April 2018.
Hadfi A., 2012, « Evaluation du pouvoir entartrant des
eaux du secteur agricole du grand Agadir », PhD
Thesis, Ibn Zohr University, Agadir, Morocco.
Lerato Lethea, 2017, « Impact of water hardness on energy
consumption of geyser heating elements », Water
SA vol.43 n.4 Pretoria Oct. 2017, South Africa.
Available at :
http://www.scielo.org.za/scielo.php?script=sci_arttext
&pid=S1816-79502017000400009. Accessed: Oct
2018.
Konstadinos Abeliotis, 2015, « Impact of water hardness
on consumers’ perception of laundry washing result in
five European countries»,International Journal of
Consumer Studies 39 , Harokopio University, Athens,
Greece. Available
at :https://onlinelibrary.wiley.com/doi/full/10.1111/ijcs
.12149 Accessed: Oct 2018.
Bruce A. Cameron, «2011, Detergent Considerations for
Consumers: Laundering in Hard Water—How Much
Extra Detergent Is Required? »,journal of extension
Volume 49 Number 4 Article Number 4RIB6,
University of Wyoming Laramie, Wyoming. Available
at :https://www.joe.org/joe/2011august/rb6.php. .
Accessed: Oct 2018.
Union française des professionnels du traitement de l’eau,
(n.d.), « Tout sur le calcaire ». Available at :
http:www.uae.fr. Accessed: August 2018.
Sante Canada, 1979, « Recommandations pour la qualité de
l'eau potable au Canada: document technique – la
dureté ». Available at : https://www.canada.ca/fr/sante-
canada/services/publications/vie-
saine/recommandations-pour-qualite-eau-potable-
canada-document-technique-durete.html. Accessed:
August 2018.
Cortial, N, (n.d.), «Précipitation - Produit de solubilité».
Available at:
http://nicole.cortial.net/complements/chimie/web-
cours-pr%E9cipitation.pdf. Accessed: May 2018.
Health misitry, 2006 « Qualité des eaux d’alimentation
humaine, Norme Marocaine homologuée », Morocco.
ICCSRE 2018 - International Conference of Computer Science and Renewable Energies
428
