
In Vitro Study of DNA Adduct 8-hydroxy-2’-deoxyguanosine (8-
OHdG) Formation Based on Fenton-like Reaction Using Chromium
(III) and Benzo[a]pyrene
Budiawan, Intan Cahaya Dani, Sri Handayani, Filia Stephanie. Ridla Bakri
Departement of Chemistry, FMIPA Universitas Indonesia, Kampus UI Depok, 16424, Indonesia
Keywords: Benzo[α]pyrene, DNA Adduct, 8-OHdG, Fenton-like Reaction
Abstract: In this research, study of 8-hydroxy-2’-deoxyguanosine (8-OHdG) caused by exposure of Chromium (III)
and Benzo[a]pyrene was conducted. This study was done by reacting 2’-deoxyguanosine as DNA base with
xenobiotic like Benzo[a]pyrene with variation of pH (7.4 and 8.4), incubation time (7 and 12 hours), and
incubation temperature (37
o
C and 60
o
C). On this mixture, another observation was conducted with addition
of Chromium (III) and H
2
O
2
as the Fenton-like reaction reagent. 8-OHdG DNA Adduct was then analyzed
with High Performance Liquid Chromatography (HPLC) reversed phase with UV detector on 245 nm
wavelength. The mixture of pH 6.7 Phospate Buffer 10mM and LC-grade methanol with ratio of 85:15 and
1 mL/minute flow rate were used in the measurement of 8-OHdG. On every mixture in all pH, time, and
temperature variation, 8-OHdG was detected with the concentration below the Limit of Quantification, thus
the concentration cannot be quantified. Addition of the Fenton-like reaction reagent also impacted on higher
8-OHdG concentration in result. Longer incubation time and higher incubation temperature were proved to
generate more 8-OHdG, meanwhile the variation of pH did not significantly affect the concentration of
generated 8-OHdG in the mixture.
1 INTRODUCTION
Benzo[a]pyrene (BaP) is a Polycyclic Aromatic
Hydrocarbon (PAH) compound. PAH is an aromatic
molecule composed of carbon and hydrogen atoms,
and consists of two or more aromatic ring molecules.
Some PAH compounds are known to have quite
high carcinogenic properties, especially those with 4
to 6 aromatic rings [1]. BaP is a PAH compound that
has the highest carcinogenic potential and is used as
an indicator of PAH contamination in the
environment. BaP can be formed through pyrolysis
and pyro synthesis. Pyrolysis is the reaction of
breaking organic matter into simple fragments, while
pyro synthesis is the reaction of the formation of
aromatic compounds from pyrolysis fragments.
Pyrolysis occurs at sufficiently high temperatures in
a dry environment or without water. BaP is formed
when the pyrolysis temperature is above 425°C [2].
Food processing such as smoking, grilling, or
roasting may lead to the increase of BaP
concentration in food, since BaP in the smoke that
originated from the woods can be easily absorbed by
the food during the process. Stolyhwo in 2005 [3]
reported that on the outside skin of the smoked fish
contained 50 μg/kg BaP. Similar research is also
conducted by Kafeelah A. in 2015 [4], which states
that in smoked fish, contained Polycyclic Aromatic
Hydrocarbons (PAHs) with a very significant
amount compared to non-smoked fish.
The exposure of a xenobiotics in the human body
can cause many variety of risks, depending on the
xenobiotic toxicity. BaP is a carcinogenic
compound, since BaP can trigger free radical
formation in the human body through Fenton /
Fenton-like reaction. Radical compound such as
radical hydroxyl can react with the forming base of
Deoxyribonucleic Acid (DNA), and result in a
conformational change in the basic structure of the
DNA. This occurence produces a new compound
called DNA adduct. This is a reversible event, in
which the DNA in the body has its regeneration
mechanism to recover its structure. However, if this
event occurs repeatedly or exposure to xenobiotics
Budiawan, ., Dani, I., Handayani, S., Stephanie, F. and Bakri, R.
In Vitro Study of DNA Adduct 8-hydroxy-2’ deoxyguanosine (8-OHdG) Formation Based on Fenton-like Reaction Using Chromium (III) and Benzo[a]pyrene.
DOI: 10.5220/0009842500002406
In Proceedings of BROMO Conference (BROMO 2018) - Symposium on Natural Product and Biodiversity, page 1
ISBN: 978-989-758-347-6
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
1

persists, it can be resulted in permanent damage to
the structure of DNA and increasing the risk of DNA
mutation on the cell (carcinogenesis) in the body of
the living organism [5].
DNA that have mutated by exposure to
xenobiotics through a series of reactions that
produce radical compounds can form one of the
DNA adduct compounds 8-hydroxy-2'-
deoxyguanosine (8-OHdG). These compounds can
come out of the cell nucleus and contained in human
blood or urine. 8-OHdG may act as a biomarker of
DNA structure modification; if the biological sample
analysis detects the presence of 8-OHdG, then it is
can be confirmed that the DNA structure has been
damaged.
The Fenton-like reaction is a formation of a
radical compound, using a metal compound beside
iron as the catalyst. In this study, Chromium (III)
were used in the in vitro reaction. Chromium is one
of the most abundant metals in nature and can be
exposed into living processes through its use in
industries such as wood preserving, and plating [6].
The presence of Chromium (III) metal becomes the
catalyst for H2O2 to form a hydroxyl radical. This
can be understood by the following series of
reactions[7]:
Cr (III) + H
2
O
2
→ Cr(IV) + HO
-
+ HO
HO + H
2
O
2
→ O
2
-
+ H+ + H
2
O
O
2
-
+ H
2
O
2
→ O
2
+ HO
-
+ HO
O
2
-
+ Cr(IV) → O
2
+ Cr(III)
2H
2
O
2
→ O
2
+ 2H
2
O
In this in vitro research, the damage of the DNA
structure as the result in the xenobiotic BaP
exposure to one of the DNA base 2’-
deoxyguanosine-5’monohydrate (2’-dG) and the
formation of 8-OHdG as a DNA Adduct were
studied. This reaction were involving the radical
hydroxyl pathway from the Fenton-Like reaction
using Chromium (III)..
2 MATERIAL AND METHODS
2.1 Material
The materials that used in the in vitro study are
2’deoxyguanosine -5’-monohydrate (Sigma
Aldrich), Hydrochloric Acid, Sodium Hydroxide,
Phospate Buffer, Acetic Buffer, Chromium (III)
Oxide (Merck), LC-Gradient Grade Methanol
(Merck), Dimethyl Sulfoxide (Merck),
Benzo[a]pyrene, 8-hydroxy-2’-deoxyguanosine
standard (Sigma Aldrich), H
2
O
2
(Merck). All
materials were used without further purification.
HPLC data were acquired using Hitachi Primaide
HPLC with YMC-TriartC18/S-5um/12nm, 250 x 4.6
mml.D reversed phase column and 254 nm UV
Detector.
2.2 Methods
2.2.1 Characterization and Method
Validation of 8-OHdG Measurement
20 μL of 8-OHdG standard with the concentration
between 10-100 ppb were injected into HPLC
column using sodium phosphate pH 6.7 buffer 10
mmol/L and methanol with the ratio of 85:15 as the
mobile phase and 1mL/minute flow rate. The results
are then plotted on the calibration curve of 8-OHdG.
8-OHdG quantification in the sample determined
from the linearity equation from the calibration
curve. Repeatability test was done by injecting 30
ppb and 80 ppb concentration of 8-OHdG standard
to the HPLC column 6 times continuously. The
repeatability then acquired by comparing the
standard peak in every measurements.
2.2.2 In Vitro Study
The DNA base used was 2'-deoxyguanosine (6 ppm)
incubated with BaP (60 ppm) under variation of
condition in pH (7.4 and 8.4), temperature (37
o
C and
60
o
C), and incubation time (7 hours and 12 hours),
then formation of 8-hydroxy-2'-deoxyguanosine (8-
OHdG) is observed. Incubation with Chromium (III)
metal (120 ppm) and H
2
O
2
(120 ppm), and with the
combination was also performed. Before analyzed,
mixture was first centrifuged for 15 minutes and
then decanted. A total of 20 μL sample were injected
into a reversed-phase HPLC column under the same
condition as in the characterization and method
validation. The results of the sample measurements
were then compared with the 8-OHdG standard
calibration curve. The quantification of 8-OHdG in
the sample was done by measuring the peak area of
the sample, then calculated using the equation from
8-OHdG standard calibration curve at various
concentrations.
3 RESULTS AND DISCUSSION
In this study, High Performance Liquid
Chromatography were used to determine the
concentration of DNA Adduct formed. Linearity test
was done to determine the detector response in the
analyte concentration alteration. This test was done
BROMO 2018 - Bromo Conference, Symposium on Natural Products and Biodiversity
2
with injecting 8-OHdG standard in a series of
concentration (10, 30, 50, 80, and 100 ppb) to the
instrument, and then the peak area plotted in the
calibration curve. Coefficient of correlation 0.9975
and the regression equation of y = 81.169x – 321.52
were obtained, and the limit of detection of 5.19 ppb
and limit of quantification 17.29 ppb also obtained
by a statistical equation. Limit of detection defined
as the concentration in which the analyte can be
confirmed as 8-OHdG, but cannot be quantified. As
for the limit of quantification, any concentration of
analyte in the sample that fall upper the limit can be
quantified statistically [8].
The repeatability test was performed to
determine the accuracy of the HPLC method used in
the measurement. This test is done by measuring the
8-OHdG standard at concentrations of 30 ppb and 80
ppb repeatedly as much as six times. One of the
values that can precipitate precision is the value of
the coefficient variation. In this research, the value
of coefficient variation for 8-OHdG standard with
concentration of 30 ppb is 1,627% and for 8-OHdG
standard with concentration 50 ppb is 0,996%. The
incubation of mixture was done under various
condition of pH (7.4 and 8.4), temperature (37
o
C and
60
o
C) and incubation time (7 and 12 hours). pH 7.4
and 37
o
C was used to be the analogue of the
physiological pH and human body temperature, a
higher pH, temperature, and the variation of
incubation time were used to characterized the DNA
Adduct formation profile. Also, pH 8.4 can be used
as an analogue of the human bile condition [9].
The stationary phase used in this study is column
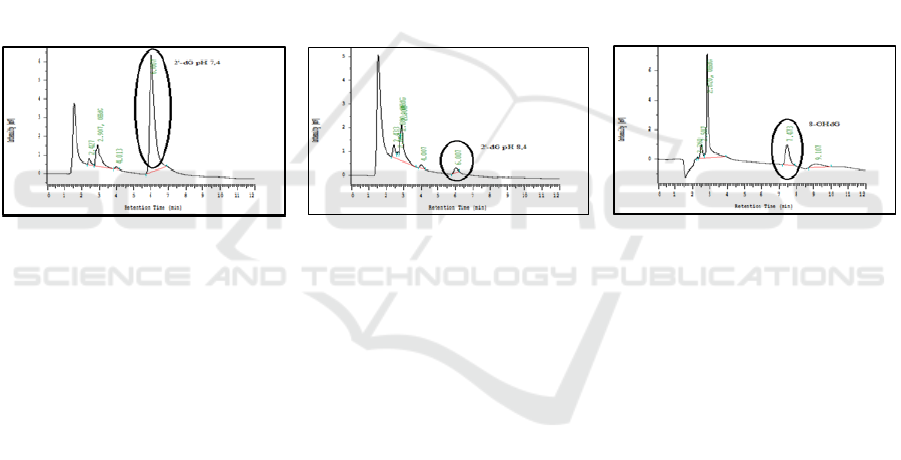
C18. In this condition the peak was obtained at a
retention time of 6,007 min for standard 2’-dG pH
7.4, 6,007 min for standard 2'-dG pH 8.4, and 7,473
min for 8-OHdG. Standard chromatogram profiles
for standard 2'-dG pH 7.4, 2’-dG pH 8.4, and 8-
OHdG can be seen in Fig. 1.
Figure 1. (a) Standard Chromatogram dG pH 7.4 6 ppm, (b) Standard Chromatogram dG pH 8.4 6 ppm, (c) Chromatogram
Standard 8-OHdG 500 ppb
The results obtained from the mixture
chromatograms show that 8-OHdG is formed at all
time, temperature, and pH variations. This suggests
that 8-OHdG can be formed at human physiological
temperatures of 37°C, and at physiological pH of
7.4, so that 8-OHdG is shown to act as a biomarker
of DNA structure damage to the human body. 8-
OHdG is considered detectable because all values
are above LOD, but the value cannot be quantified
because it is below the LOQ.
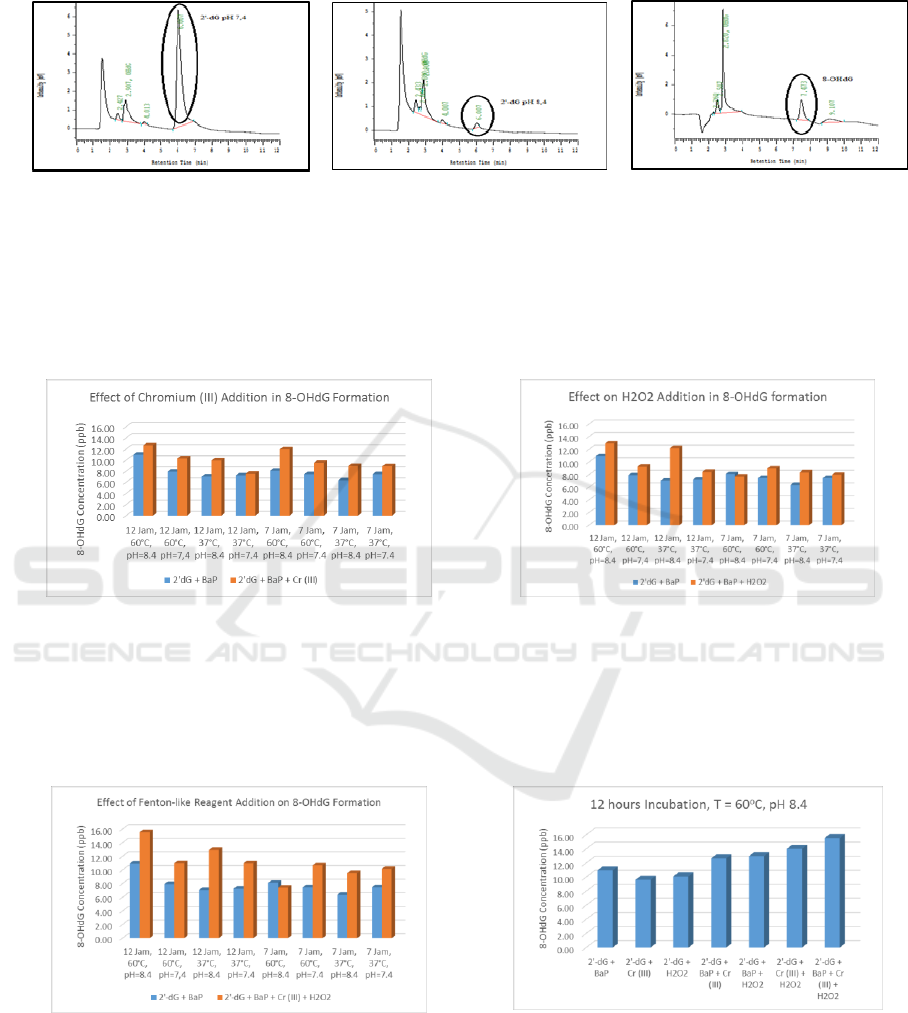
To observe the effect of metal addition in the DNA
Adduct formation on 2'-dG, whether Cr (III) react
independently in the formation of 8-OHdG or
synergistic with the xenobiotic (in this case BaP),
incubation of the 2'-dG and metal mixture Cr (III)
was done. Although the pathway and its formation
mechanisms are not yet known, on the measurement
results, it is concluded that Cr (III) and 2'-dG can
form DNA Adduct. This indicates that the effect in
addition of Cr (III) metal in the BaP and 2'-dG
mixture can increase the concentration of the 8-
OHdG. This statement corresponds to the
concentrations obtained from the 2'-dG and BaP
mixtures, the result of most of the mixtures
indicating that the obtained 8-OHdG concentrations
were greater in the 2-dG, BaP, and Cr (III) mixtures.
This can be due to the addition of Cr (III) to give a
synergistic effect with BaP because Cr (III) can also
damage the DNA structure and produce DNA
Adduct (Fang et. al, 2014)[10].
The increase in 8-OHdG concentration in H
2
O
2
addition can be correlated by the fact that H
2
O
2
is a
strong oxidant which can then be reduced to a
hydroxyl radical (•OH) [7] with the reaction:
O
2
•
−
+ H
2
O
2
→ OH• + OH
-
+ O
2
Cr
3+
+ H
2
O
2
→ Cr
6+
+ OH• + OH
-
To analyze the effect of H
2
O
2
addition in 8-
OHdG formation, it can be done by comparing the
results of 8-OHdG concentrations obtained on
mixtures using H
2
O
2
and mixtures not using H
2
O
2
.
For the effect on the addition of metal and H
2
O
2
as
oxidizing agent can be seen in Figure 2.
In Vitro Study of DNA Adduct 8-hydroxy-2’ deoxyguanosine (8-OHdG) Formation Based on Fenton-like Reaction Using Chromium (III)
and Benzo[a]pyrene
3

Figure 2. (a). The Effect of Metal Addition (b). The Effect
on H
2
O
2
Addition
Theoretically, the occurrence of Fenton-like
reactions can trigger the formation of more radical
compounds so that more DNA adducts are formed.
From the result, it can be seen that the addition of
Fenton-like reaction reagent increase 8-OHdG
formation results on average as much as 40.9%. The
highest concentration of 8-OHdG was formed in this
mixture, compared to the addition of metal (average
concentration increase of 21.4%), or addition of
H
2
O
2
(average concentration increase 27.5%). The
mixture between 2'-dG, BaP, Cr (III), and H
2
O
2
becomes a mixture which form 8-OHdG with the
highest concentration in all variations. This
correlation can be seen in figure 3.
Figure 3. (a) The Effect of Fenton Reagent Addition (b) 8-
OHdG Formation under variation of condition
Incubation of mixture at higher temperatures and
longer incubation time yields 8-OHdG with higher
concentrations. This is because increasing
temperature will increase the kinetic reaction, and
longer incubation time will increase the duration of
collisions between reagents molecule. But changes
in pH were observed to have little effect on the
formation of 8-OHdG. The correlation can be seen
on figure 4.
4 CONCLUSIONS
8-OHdG DNA Adduct formed in the mixture was
detected on 6.5-7.1 minute retention time in the
instrument. Reaction between 2’-deoxyguanosine
and BaP under various condition and addition of the
Fenton-like reagent resulted in 8-OHdG formation
with the concentration above the LOD, but below
the LOQ, so the value cannot be quantified.
Addition of metal (Chromium (III)) and strong
oxidation agent (H2O2) increase the concentration
of 8-OHdG in the sample mixture. The mixture
between 2’-dG, BaP, and the Fenton-like reagent
yield the highest concentration of DNA Adduct
detected than other mixture. Reaction on 60oC and
12 hours incubation time increasing the 8-OHdG
concentration.
ACKNOWLEDGEMENTS
Authors wishing to thank PITTA-DRPM UI for
funding this project and Balai K3 Hiperkes for
facilitating the HPLC instrument.
BROMO 2018 - Bromo Conference, Symposium on Natural Products and Biodiversity
4

REFERENCES
Luch, A., & Baird, W. (2005). Metabolic Activation and
Detoxification of Polycyclic Aromatic Hydrocarbons.
The Carcinogenic Effects Of Polycyclic Aromatic
Hydrocarbons, 19-96.
Guillén, M., Sopelana, P., & Partearroyo, M. (2000).
Polycyclic Aromatic Hydrocarbons in Liquid Smoke
Flavorings Obtained from Different Types of Wood.
Effect of Storage in Polyethylene Flasks on Their
Concentrations. Journal Of Agricultural And Food
Chemistry, 48(10),
Stołyhwo, A., & Sikorski, Z. (2005). Polycyclic aromatic
hydrocarbons in smoked fish – a critical review. Food
Chemistry, 91(2), 303-311.
Kafeelah, A., Lucy, N., Kafayat, A., Shehu, L., Julius, I.,
& Titus, O. (2015). Influence of fish smoking methods
on polycyclic aromatic hydrocarbons content and
possible risks to human health. African Journal Of
Food Science, 9(3), 126-135.
Briedé, J., Godschalk, R., Emans, M., de Kok, T., van
Agen, E., & van Maanen, J. et al. (2004). In Vitro and
In Vivo Studies on Oxygen Free Radical and DNA
Adduct Formation in Rat Lung and Liver during
Benzo[a]pyrene Metabolism. Free Radical Research,
38(9), 995-1002.
Metze, D., Jakubowski, N., Klockow, D., (2005).
Speciation of chromium. In: Cornelis, R., Crews, H.,
Caruso, J., Heumann, K.G. (Eds.), Handbook of
Elemental Speciation II: Speciation in the
Environment, Food, Medicine & Occupational Health.
John Wiley & Sons, Ltd.
Nixon Ogendi Mwebi, (2005). Fenton & Fenton-Like
Reactions: Nature of Oxidizing Intermediates.
University of Maryland, College Park
Riyanto. (2014). Validasi & Verifikasi Metode Uji: Sesuai
dengan ISO/IEC 17025 Laboratorium Pengujian dan
Kalibrasi. Yogyakarta: Deepublish, Ed.1, Cet. 1:66
Sutor, D. J., & Wilkie, L. I. (1976). Diurnal variations in
the pH of pathological gallbladder bile. Gut, 17(12),
971–4.s
Fang, Z., Zhao, M., Zhen, H., Chen, L., Shi, P., & Huang,
Z. (2014). Genotoxicity of Tri- and Hexavalent
Chromium Compounds In Vivo and Their Modes of
Action on DNA Damage In Vitro. Plos ONE, 9(8),
e103194. Moore, R., Lopes, J., 1999. Paper templates.
In TEMPLATE’06, 1st International Conference on
Template Production. SCITEPRESS.
Smith, J., 1998. The book, The publishing company.
London, 2
nd
edition.
In Vitro Study of DNA Adduct 8-hydroxy-2’ deoxyguanosine (8-OHdG) Formation Based on Fenton-like Reaction Using Chromium (III)
and Benzo[a]pyrene
5
Attachment
Figure 1
(a)
(b)
(c)
Figure 1. (a) Standard Chromatogram dG pH 7.4 6 ppm, (b) Standard Chromatogram dG pH 8.4 6 ppm, (c) Chromatogram
Standard 8-OHdG 500 ppb
Figure 2
(a)
(b)
Figure 2. (a). The Effect of Metal Addition (b). The Effect on H
2
O
2
Addition
Figure 3
(a)
(b)
Figure 3. (a) The Effect of Fenton Reagent Addition (b) 8-OHdG Formation under variation of condition
BROMO 2018 - Bromo Conference, Symposium on Natural Products and Biodiversity
6
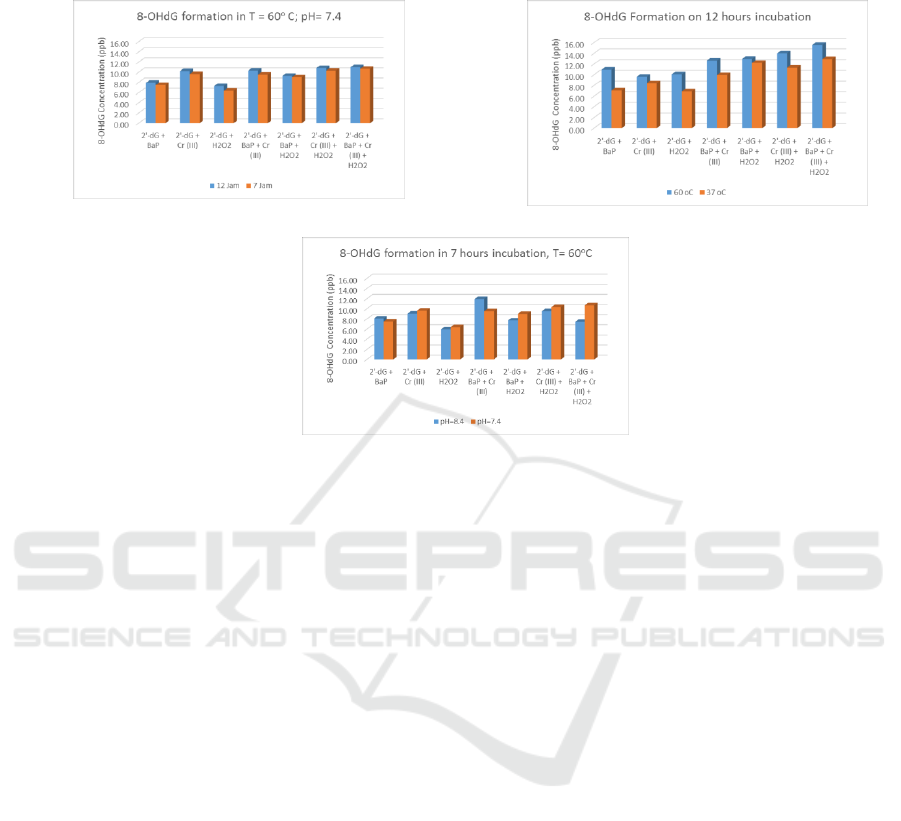
Figure 4
(a)
(b)
(c)
Figure 4. (a) Effect on Incubation time variation (b) Effect on Temperature variation (c) Effect on pH variation
In Vitro Study of DNA Adduct 8-hydroxy-2’ deoxyguanosine (8-OHdG) Formation Based on Fenton-like Reaction Using Chromium (III)
and Benzo[a]pyrene
7
