
Study on Biological Active Components of Eurycoma Longifolia
Nik Nur Shamiha N. D
1,3*
, Siti Shukriyah S.
1,2
, Ryuichiro S.
2
, Yoshiaki S.
2
, Jiyauddin K.
1,3
, Ibrahim
A.
1,3
*, Mohd Nizam A. G.
1,3
, Mohd Fadli A.
1,3
and Eddy Y.
3
1
School of Pharmacy, Management and Science University, Selangor Darul Ehsan, Malaysia
2
Faculty of Pharmaceutical Sciences, Josai University, 1-1 Keyakidai, Sakado, Saitama 350-0295, Japan
3
ICHLAS, Management & Science University, Selangor, Malaysia
Keywords: Eurycoma longifolia, Anti-glycation activity, % of inhibition, Tongkat Ali, AGEs formation inhibition in
vitro.
Abstract: Background and aims: Constant hyperglycemia in diabetic patient may lead to excess glycation and thus is
believed to cause diabetic complications. Eurycoma longifolia (Simaroubaceae) is tested for inhibitory
activity of advanced glycation end-products (AGEs) formation in vitro. Materials and methods: Three
concentration of methanolic extract were tested together with bovine serum albumin in anti-CML antibody.
HRP- conjugated anti-mouse IgG antibodies were introduced and sample were reacted with phenyldiamine
dihydrochloride. Absorbance were read by using micro-ELISA and percentage of inhibition were
calculated. Results: The calculated percentage of AGEs formation inhibition by E. longifolia root are -3.62
% (0.1 mg/mL), 58.38 % (1 mg/mL) and 92.28 % (10 mg/mL) as compared to aminoguanidine 5.55 % (0.1
mg/mL), 39.32 % (1 mg/mL), 72.92 % (10 mg/mL) as referring to the concentration. Since the biological
activity was tested on the whole methanolic extract, the activity is suggested to be due to synergistic activity
of the extract. Conclusion: New biological activity of E. longifolia methanolic extract which is inhibition of
AGEs formation in vitro is seen. However, isolation of Fr.8-2, m/z:381 does not lead to any compound
isolated related to the plant.
1 INTRODUCTION
This study focus on the antiglycation activity of
Eurycoma longifolia (Simaroubaceae). To date,
there is no known activity on inhibition of advanced
glycation end products (AGEs) formation by E.
longifolia. The plant is widely known for anti-tumor
promoting activities, antischistosomal,
plasmodicidal activities (Jiwajinda, S. et al., 2002),
potent antiulcer activity (Tada, H. et al., 1991), helps
to improves stress hormone profile and certain mood
state parameters (Talbott, S. M., Talbott, J. A.,
George, A., & Pugh, M., 2013), cytotoxic activity
(Kuo, P.C., Damu, A.G., Lee, K.H., & Wu, T.S.,
2003), antibacterial action (Farouk, A., & Benafri,
A., 2007) as well as antimalarial activity (Ang, H.,
Chan, K., & Mak, J., 1995). Chronic use of E.
longifolia is said to increase the testosterone level in
men.
AGEs formation lead to kaput protein. AGEs
formation in normal healthy people may not be
detrimental to their health compared to diabetic
patient. It is believed that major complication of
diabetes; nephropathy, neuropathy and retinopathy
are augmented by AGEs formation as well as
constant hyperglycemia. This study utilizes the
method of evaluation based on a previous study
conducted by Okada, Y., Ishimaru, A., Suzuki, R.
and Okuyama, T. in 2004. Inhibition of AGEs
formation is based on the carboxymethyl lysine level
inhibition.
By determining the biological activity, it may
leads to isolation of compounds responsible for the
said activity. This study suggest for further progress
in formulating therapeutic agents to counter diabetic
complications and thus improve the patients’ quality
of life and reduce the mortality rate among diabetic
due to complications.
Shamiha, N., Shukriyah, S., S., R., S., Y., Khan, J., A., I., Nizam, M., Asmani, M. and Yusuf, E.
Study on Biological Active Components of Eurycoma Longifolia.
DOI: 10.5220/0009846400002406
In Proceedings of BROMO Conference (BROMO 2018) - Symposium on Natural Product and Biodiversity, page 1
ISBN: 978-989-758-347-6
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
1

2 MATERIALS AND METHODS
2.1 Materials
E. longifolia sliced, dried roots were procured from
Malaysia, 99.5 % Methanol (Wako Pure Chemical
Industries, LTD, Lot: DSH3947), 99.9 % Methanol
(Wako Pure Chemical Industries, LTD, Lot:
DSJ0787), 99.7 % Methanol (Wako Pure Chemical
Industries, LTD, Lot: DSF3711), ethyl acetate
(Kanto Chemical Co. Inc. Lot: 51B1137), 99.0 % 1-
butanol (Wako Pure Chemical Industries, LTD, Lot:
DSR6754), 99.0 % Chloroform (Wako Pure
Chemical Industries, LTD, Lot: DSF3237), 99.8 %
Chloroform D + Silver Foil (Cambridge Isotope
Laboratories, Inc., Lot: PR-27572/04086CL1),
dimethyl sulfoxide, bovine serum albumin,
phosphate buffer solution, glucose, PBS containing
0.05% Tween 20, 0.5% gelatin in 100 mL coating
buffer, HRP-conjugated anti-mouse IgG antibodies,
1,2-phenyldiamine dihydrochloride, 100 µL of 1 M
sulfuric acid.
2.2 Apparatus
Evaporating flask, mantel heater, rotary evaporator
(EYELA NVC, No: 038006204), desiccator with
silica gel, TLC ODS plate, TLC silica gel plate. UV
light transmitter, microtube, micropipette, 96-well
plate, micro-ELISA plate reader, HPLC-RI detector
(Waters 600 Pump, Waters 600 Controller, Shodex
RI-201H Refractive Index Detector), MPLC Micro
pump KPW-20 (Kusano, Kagakukikai Co.),
Advantec Fraction Collector (CHF122SC), H-NMR
Varian (Agilent, 400 Hz), EI-MS (JEOL), HPLC
ODS-4151-N column (Senshu Pak, 10 x 150 mm,
No: 1110201H), MPLC column (MERCK,
LiChroprep Si 60 (40-63 µm), No: 540087666).
2.3 Preparation of Methanolic Extract
Methanolic extract of the roots of E. Longifolia (200
g) was obtained by extraction with MeOH (5.4 L)
three times under reflux for 3 hours. The solvent was
evaporated in vacuo to give MeOH extract (6.74 g).
2.4 Isolation of Components from
Methanolic Extract
The methanolic extract was suspended in water, then
extracted with EtOAc and n-BuOH, sequentially.
Each soluble portion was evaporated in vacuo to
give EtOAc (1.07 g) and n-BuOH (1.79 g) fractions,
respectively. The EtOAc fraction was
chromatographed on a prepacked silica gel column
(LiChroprep Si60 (40-63 µm) Merck Co. serial
number: 540087666, 140987) eluting with CHCl
3
to
give 15 fractions. Fr.8, Fr.9, and Fr.10 was further
purified with HPLC-RI (Detector: RI-201H,
SHODEX, Column: ODS-4151-N; size: 10 x 150
mm; number: 1110201H, Senshu Scientific Co. Ltd.)
detector. Fr.8 (0.0123 g) was further purified with
HPLC-RI detector using MeOH to provide four
fraction Fr.8-1 (0.0001 g) Fr.8-2 (0.0006 g) Fr.8-3
(0.0001 g) and Fr.8-4 (0.0001 g). Fr.9 (0.0148 g)
was further purified with MeOH-H
2
O mixture
(MeOH : H
2
O = 10 : 1) to gives four fraction Fr.9-1
(0.0014 g) Fr.9-2 (0.0010 g) Fr.9-3 (0.0014 g) and
Fr.9-4 (0.0007 g). Fr.10 (0.0074 g) was further
purified with MeOH-H
2
O mixture (MeOH : H
2
O =
10 : 1) to gives three fraction Fr.10-1 (0.0002 g)
Fr.10-2 (0.0003 g) Fr.10-3 (0.0001 g).
2.5 Inhibition Test on AGE Formation
in Vitro
BSA was incubated with 200 mmol/L glucose in
both presence and absence of test compound for 7
days in 0.1 M of phosphate buffer (pH 7.4) at 37 °C.
After incubation, coating buffer, blocking buffer and
anti-CML antibody were introduced to the cell.
HRP-conjugated anti-mouse IgG antibodies was
treated to the cells. 1 M sulfuric acid was used to
stop the reaction. The level of inhibition is measured
by calculating the level of CML measured by CML-
specific micro-ELISA plate reader at 492 nm
(SpectraMax PLUS 190PC ROM v1.23). Percentage
of inhibition was calculated as in following
equation:
Inhibition (%) = [1-(A
s
– A
b
)/(A
c
– A
b
)] x 100,
where A
s
is the CML level in the incubated mixture
with sample, A
c
is the CML level in the incubated
mixture without sample, and A
b
is the CML level in
the incubates mixture without sample and glucose
that served as blank control.
3 RESULTS
Methanolic extraction of E.longifolia root (200 g)
yielded 6.74 g of dried extract. Repeated extraction
under reflux ensures complete extraction from the
root. 20 mg of methanolic extract were subjected to
inhibition of AGEs formation in vitro by measuring
CML level using microELISA at 492 nm. Three
reading were recorded for each concentration. The
average reading is tabulated in Table 1.
BROMO 2018 - Bromo Conference, Symposium on Natural Products and Biodiversity
2
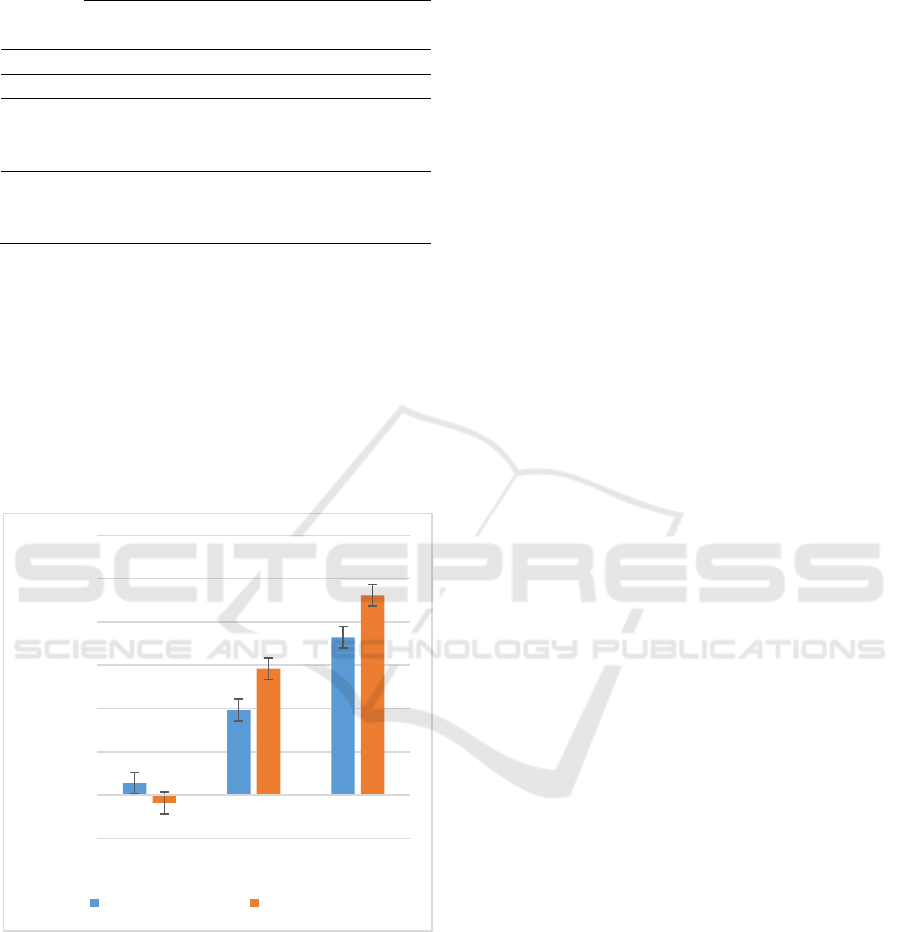
Table 1: Average reading of absorbance of AGEs
inhibition by microELISA.
Sample
Reading
1
Reading
2
Reading
3
Average
reading
Blank
0.755
0.765
0.663
0.728
Control
2.592
2.280
2.287
2.386
AG 0.1
2.522
2.240
2.119
2.294
AG 1
1.657
1.758
1.787
1.734
AG 10
1.260
1.069
1.201
1.177
EL 0.1
3.146
2.220
1.972
2.446
EL1
1.344
1.683
1.226
1.418
EL 10
0.842
0.993
0.733
0.856
Upon calculating the average reading of each
sample, the percentage of AGEs formation inhibition
were determined by substituting the absorbance
obtained by Micro-ELISA plate reader in the
formula. -3.62 %, 58.38 % and 92.28 % of inhibition
were calculated in E. longifolia with concentration
of 0.1 mg/mL, 1 mg/mL and 10 mg/mL respectively.
The percentage of inhibition of AGEs by E.
longifolia compared to Aminoguanidine is as in
Figure 1.
Figure 1: Comparison between percentages of inhibition
of AGEs formation between aminoguanidine (positive
control) and E. longifolia root extract.
4 DISCUSSION
Three concentration of E. longifolia root were
tested; 0.1 mg/mL, 1 mg/mL, and 10 mg/mL. The
calculated percentage of AGEs formation inhibition
by E. longifolia root are -3.62 % (0.1 mg/mL), 58.38
% (1 mg/mL) and 92.28 % (10 mg/mL) as compared
to aminoguanidine 5.55 % (0.1 mg/mL), 39.32 % (1
mg/mL), 72.92 % (10 mg/mL) as referring to the
concentration. The activity of E. longifolia is
suggested to match the activity of aminoguanidine.
The percentage of inhibition is concentration
dependent. Since the biological activity was tested
on the whole methanolic extract, the activity is
suggested to be due to synergistic activity of the
extract. Due to the small amount of root, the isolated
fractions yield are very small. Thus, we are unable to
test the biological activity on each fraction. Based on
EI-MS analysis of Fr.8-2, the molecular weight is
suggested to be m/z: 381 as attached in Appendix.
However, based on this data alone, we are not able
to relate the finding to any compounds reported to be
having relationship with the plant E. longifolia.
5 CONCLUSIONS
Methanolic extract of E. longifolia is suggested to be
having the inhibitory activity of AGEs formation.
The antiglycation activity is believed to be
synergistic action between compounds in the extract.
Further isolation of fractions however does not lead
to identification of isolates. In the future study, it is
strongly suggested that large amount of plant sample
should be used and care attention to work procedure
must be applied to prevent possible impurity to the
components.
ACKNOWLEDGEMENTS
The authors would like to thank all members of
School of Pharmacy MSU and Josai university for
their support.
REFERENCES
Ang, H., Chan, K., & Mak, J. (1995). In Vitro
Antimalarial Activity of Quassinoids from
Eurycoma longifolia against Malaysian
Chloroquine-Resistant Plasmodium falciparum
Isolates. Planta Med Planta Medica, 61(02),
177-178. doi:10.1055/s-2006-958042
Farouk, A., & Benafri, A. (2007). Antibacterial
Activities of Eurycoma Longifolia A Malaysian
medicinal plant. Saudi Med J, 28(9), 1422-1424
-20
0
20
40
60
80
100
120
0.1 mg/mL 1 mg/mL 10 mg/mL
Percentage (%) of inhibition
Concentration
Aminoguanidine E. longifolia
Study on Biological Active Components of Eurycoma Longifolia
3

Jiwajinda, S., Santisopasri, V., Murakami, A.,
Kawanaka, M., Kawanaka, H., Gasquet,
M., Ohigashi, H. (2002). In vitro anti-tumor
promoting and anti-parasitic activities of the
quassinoids from Eurycoma longifolia, a
medicinal plant in Southeast Asia. Journal of
Ethnopharmacology, 82(1), 55-58.
doi:10.1016/s0378-8741(02)00160-5
Kuo, P.C.; Damu, A.G.; Lee, K.H.; Wu, T.S. (2003)
Cytotoxic and antimalarial constituents from the
roots of Eurycoma longifolia. Biorg. Med.
Chem.,12, 537–
Okada, Y., Ishimaru, A., Suzuki, R., & Okuyama, T.
(2004). A New Phloroglucinol Derivative from
the Brown Alga Eisenia bicyclis : Potential for
the Effective Treatment of Diabetic
Complications. Journal of Natural Products,
67(1), 103-105. doi:10.1021/np030323j
Tada, H., Yasuda, F., Otani, K., Doteuchi, M.,
Ishihara, Y,. Shiro, M., (1991). New antiulcer
quassinoids from Eurycoma longifolia. European
Journal of Med. Chem., 26(3): 345-349.
doi:10.1016/0223-5234(91)90069-Y
Talbott, S. M., Talbott, J. A., George, A., & Pugh,
M. (2013). Effect of Tongkat Ali on stress
hormones and psychological mood state in
moderately stressed subjects. J Int Soc Sports
Nutr Journal of the International Society of
Sports Nutrition, 10(1), 28. doi:10.1186/1550-
2783-10-28
BROMO 2018 - Bromo Conference, Symposium on Natural Products and Biodiversity
4

APPENDIX
Study on Biological Active Components of Eurycoma Longifolia
5
