
Chemical Activation of Lignite by using a Combination of H
3
PO
4
-
NaHCO
3
Alwathan
1
, Muh. Irwan
1
, Panji Satrio Utomo
1
and Yuli Patmawati
1
1
Department of Chemical Engineering, Politeknik Negeri Samarinda
Jl.Dr.Cipto Mangunkusumo, Kampus Gn.Lipan Po.Box 1341 Samarinda, Kalimantan Timur - Indonesia
Keywords: Activated Carbon, Activation, Coal, Lignite, Low-Rank Coal.
Abstract: Coal is divided into four
classes: lignite, sub-bituminous, bituminous, and anthracite. Lignite is a low
rank coal. About 30% of Indonesia's coal reserves are included in the low rank category. The use of low-
rank coal is still limited for briquettes and as fuel for electricity generation. Improving the economic and
usage values of low-rank coal, processing low-rank coal into activated carbon should be done because coal
has a high carbon content.
The purpose of this study is to determine the effect of activation time on the
characteristics of the activated carbon produced by chemical activation process of lignite use a combination
of activator H
3
PO
4
- NaHCO
3
. Lignite has been prepared -100 +120 mesh is carbonized at 600
0
C for 3 h,
then after cold it was activated using 2.5 M concentration of H
3
PO
4
-NaHCO
3
for 2, 4, 6, 8 and 12 h.
Furthermore, proximate and iodine adsorption number analysis were used to
investigate the
characteristics of activated carbon produced refers to Indonesian National Standard (SNI 06-3730-1995)
including moisture content, ash content, volatile matter, fixed carbon and iodine absorption number. The
best results were obtained at 6 h of activation with the characteristics of activated carbon such as moisture
content, ash content, volatile matter, fixed carbon and iodine absorption number respectively as follows
3.5%, 14.91%, 9.81%, 71.78% and 505.1 mg/g. Activated carbon is a well known material that is used
extensively in industrial purification and
chemical recovery operations. It offers an attractive and
inexpensive option for removal of several
solutes from aqueous solutions.
1 INTRODUCTION
Coals are raw materials for many chemical syntheses
as well as cost-effective fuels for power plants due
to their low cost; however, some coals such as
low-rank coal (lignite) contain high amounts of
moisture (Karthikeyan and Mujumdar, 2009).
Activated carbon is a well known material that
is used extensively in industrial purification and
chemical recovery operations. It offers an attractive
and inexpensive option for removal of several
solutes from aqueous solutions.
Activated carbon
can be produced from different sources, such as
lignocellulosic materials, coal, baggase ash,
activated sludge and others (Shawabkeh and Al-
Ghamdi, 2014). Coal has the potential as a raw
material to produce activated carbon because it has a
high carbon content (Speight, 1994).
Activation processes are mainly categorized
into
two categories for the preparation of
activated carbon
i.e. physical and chemical
activation. Physical
activation usually involves the
carbonization of pre-cursor followed by the
gasification of the resulting char or direct
CO
2
/steam activation of the starting material.
Chemical activation involves the impregnation of
the given precursor with
activation agent such as
phosphoric acid (H
3
PO
4
), chloric acid ( H C l ) ,
nitrit acid ( H N
3
), zinc
chloride (ZnCl
2
), alkaline
metal compounds and salt.
The adsorption capacity of activated carbon is
very important because this property determines
how much of the substance can be absorbed per
gram of carbon. The activator type directly affects the
micropore structure,
specific surface area and pore
volume of the activated carbon, which makes its
adsorption capacity vary obviously (Bilal, 2016).
Activated carbon is sold at a high enough price if the
adsorption capacity is large. The quality
requirements for activated carbon refers to
Indonesian National Standard (SNI 06-3730-1995)
with max.15% moisture content, max.10% ash
182
Alwathan, ., Irwan, M., Satrio Utomo, P. and Patmawati, Y.
Chemical Activation of Lignite by using a Combination of H3PO4-NaHCO3.
DOI: 10.5220/0010025000002905
In Proceedings of the 8th Annual Southeast Asian International Seminar (ASAIS 2019), pages 182-185
ISBN: 978-989-758-468-8
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
content, max.25% volatile matter, min.65% fixed
carbon and min. 750 mg/g iodine adsorption number
(Departemen Perindustrian dan Perdagangan, 2003).
Many studies regarding the change in
adsorption capacity of activated carbon as a
function of the type of chemicals and activating
conditions have been reported.
Research
conducted by Rahim and Indriyani using 5% NaOH
activator solution produced activated carbon
according to SII standard 0258-79 even though the
ash content was still above the standard (Rahim and
Indriyani, 2010). Another study was making of
activated carbon from sengon wood with chemical
activation using NH
4
HCO
3
activator solution of
concentration variation 0; 0.5; 1; 3; 5 and 10% by
weight, produce activated carbon with moisture
content of 6.39%, ash content of 9.15%, volatile
matter of 8.81%, fixed carbon of 82.04% and iodine
absorption number of 1154.4 mg/g. Meanwhile
research with chemical activation of bituminous coal
(Kusdarini and Ghafarunnisa, 2017) used a
combination of H
3
PO
4
- NH
4
HCO
3
activator solution
(concentration 2 M - 2.5 M) for 8 h followed by
physical activation to produce activated carbon with
a moisture content of 7.5%, ash content of 9%,
volatile matter of 43.3%, fixed carbon of 40.2% and
increased iodine absorption number to 1172.56 –
1238.544 mg/g (Kusdarini and Ghafarunnisa, 2017).
The purpose of this study is to determine the
effect of activation time on the characteristics of the
activated carbon produced by chemical activation
process of lignite use a combination of activator
H
3
PO
4
- NaHCO
3
.
2 METHODOLOGY
Lignite has been prepared -100 +120 mesh is
carbonized at 600
0
C for 3 h, then after cold it was
activated using 2.5 M concentration of H
3
PO
4
-
NaHCO
3
for 2, 4, 6, 8 and 12 h. Furthermore,
proximate and iodine absorption number analysis of
activated carbon refers to Indonesian National
Standard SNI 06-3730-1995 was carried out
including moisture content, ash content, volatile
matter and fixed carbon. Table 1 summerizes the
characterization of lignite before activation.
Table 1: Characteristics of Lignite.
Parameter Value
Moisture Content, % 37.86
Ash Content, % 5.53
Volatile Matter, % 25.06
Fixed Carbon, % 31.55
Iodine Number, mg/g 215.75
Caloric Value, cal/g 3665
3 RESULT AND DISCUSSION
Table 2 summarizes the results of chemical
activation of lignite by using a combination of
H
3
PO
4
- NaHCO
3
activators.
Table 2: Characteristics of Activated Carbon.
Param
eter,%
Time, hours
2 4 6 8 12
Moist.
Cont.
5.15 3.89 3.05 3.83 3.62
Ash
Cont.
11.07 13.98 14.91 13.89 13.85
Vol.
Matter
10.36 9.71 9.81 12.03 10.08
Fixed
Crbon
73.42 72.42 71.78 70.25 72.45
Iodine
Numb
er,
mg/g
479.2 492.1 505.1 492.1 453.3
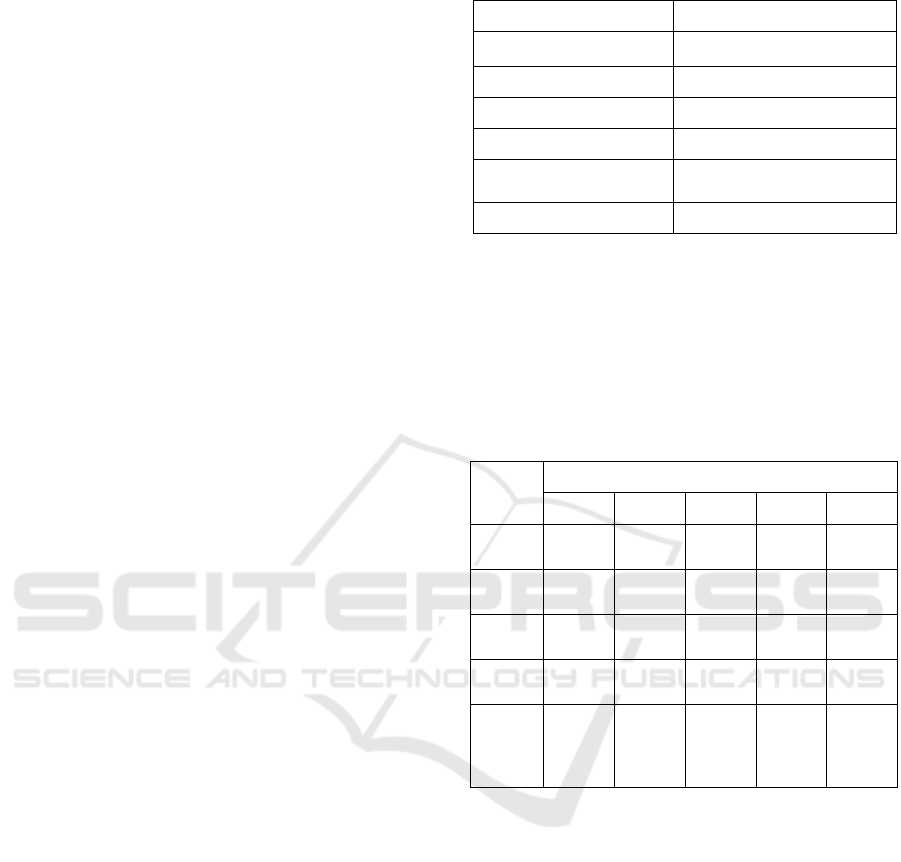
The effect of activation time on
characteristics
of activated carbon
is
represented in Figure 1 and
2.
In Figure 1 can be seen that the longer time of
activation, the moisture content has decreased from
37.86% to 3.5% at 6 h
of activation time
. This is
because water trapped into the cavity of activated
carbon will be more dehydrated by combination of
activator H
3
PO
4
- NaHCO
3
. The increase in moisture
content at 8 h and 12 h
of activation time
was due
to the hygroscopic characteristic of the activated
carbon so that in the cooling process water vapor in
the air is absorbed into the pores (Bilgen, 2016).
Chemical Activation of Lignite by using a Combination of H3PO4-NaHCO3
183
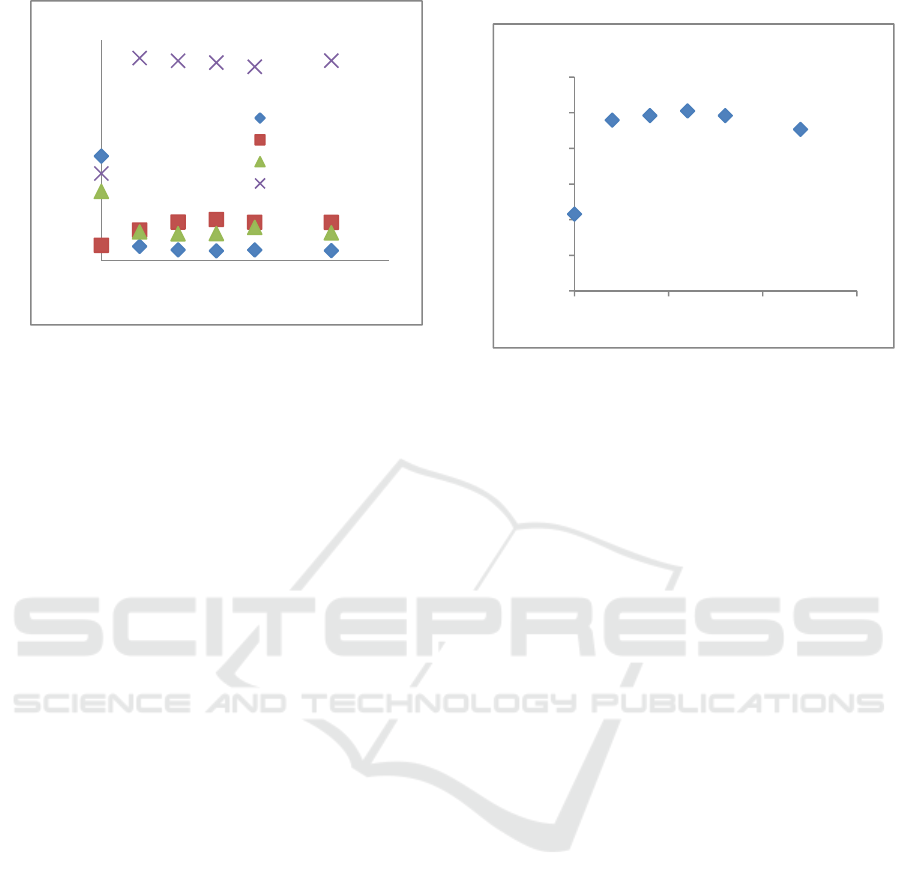
Figure 1: Characteristics of activated carbon versus time
of activation.
Ash content represents the bulk of the mineral
matter
in the coal after losing the volatile
components such as CO
2
, SO
2
, and H
2
O.
The
ash
content in Figure 1
was
measured to be 11.07%
; 13.98% ; 14.91% ; 13.89% and
13.85% at 2, 4, 6,
8 and 12 h
of activation time,
respectively.
The
increase in ash content until 6 h
of activation time
was due to the presence of alkali elements which are
absorbed in the low-rank coal pore during
immersion with an activator H
3
PO
4
- NaHCO
3
to
form a silicate from the alkali elements.
After
showing its highest value at 6 h activation time,
the ash content gradually decreased with
activation time and was 13.85% at 12 h of
activation.
Volatile matter show the portion of coal that is
released as gases and volatile liquids when heated
in the
absence of air at prescribed conditions. The
decrease in volatile matter from 25.06% until
10.36% at 2 h
of activation time
but the longer
time of activation (2 h – 6 h) of low- rank coal does
not have a significant effect on volatile matter. At 8
h activation, the volatile matter has increased
because the decomposition of H
2
CO
3
into H
2
O and
CO
2
. After 8 h activation, the volatile matter
decreased and was 10.08%
at 12 h of activation.
Fixed carbon is composed of carbon with
lesser amounts of H, N,
and S. It is generally
described as a coke-like residue. It
can be used to
give a forecast of heating value of the coal
(Speight, 2013a). Activation of lignite by using
combination of H
3
PO
4
- NaHCO
3
activators has
succeeded in increasing the fixed carbon from
31.55% to 70.25% -73.42%. This can be seen in
Figure 1. The increase in fixed carbon was due to
decrease in moisture content and volatile matter,
while the ash content does not contribute too much.
Figure 2: Iodine absorption number of activated carbon
versus time of activation.
The iodine adsorption number reflected the adsorption
per-formance of activated carbon, as shown in
Figure 2. It tends to increase from 215.75 mg/g to
505.1 mg/g at 6 h of activation time. Furthermore at
8 and 12 h of activation time there was decrease
until finally obtained an iodine absorption number of
453.3 mg/g. I
n general, the iodine
absorption
number
of activated carbon, which represents
its adsorption
capacity. When activation was
carried out longer than 6 h the decrease in
iodine
absorption number
probably
due to higher
ash content so that inorganic substances left on the
activated carbon cover the pores of the activated
carbon.
4 CONCLUSION
1.
Chemical activation of lignite by
using
a
combination of H
3
PO
4
- NaHCO
3
activators
was evaluated taking activation
time as the major influential parameters
were obtained the best condition at 6 h of
activation time with the characteristics of
activated carbon such as moisture content,
ash content, volatile matter, fixed carbon
and iodine absorption number respectively
as follows 3.5%, 14.91%, 9.81%, 71.78%
and 505.1 mg/g.
2.
Ash content and iodine absorption number
was still below the standards referred to
Indonesian National Standard (SNI 06-
3730-1995).
0
10
20
30
40
50
60
70
80
0 5 10 15
Percentage, %
Time, hours
Moisture Content
Ash Content
Volatile Matter
Fixed Carbon
0
100
200
300
400
500
600
0 5 10 15
Iodine Adsorption Number, mg/g
Time, hours
ASAIS 2019 - Annual Southeast Asian International Seminar
184

REFERENCES
Bilal Khalid et.al., 2016. Effects of KOH Activation on
Surface Area, Porosity and Desalination
Performance
of Coconut Carbon Electrodes. Desalination and
Water Treatment Journal
57. pp. 2195–2202.
Bilgen, S., 2016. The effects of Chemical Characteristics of
Coal on Coal-Based Industry. Energy Sources, Part A:
Recovery, Utilization and Environmental Effects.
38:22.
pp.
3324-3331.
Departemen Perindustrian dan Perdagangan, 2003. Syarat
Mutu dan Uji Arang Aktif SNI No. 06-3730-1995.
Balai Perindustrian dan Perdagangan.
Karthikeyan, M., Zhonghua, W. and Mujumdar, S.Arun,
2009.
Low-Rank Coal Drying Technologies—
Current Status and New Developments.
Drying
Technology: An International Journal.
Kusdarini, E., Budianto, A. and Ghafarunnisa, 2017.
Produksi Karbon Aktif dari Batubara Bituminus
dengan Aktivasi Tunggal H
3
PO
4
, Kombinasi H
3
PO
4
-
NH
4
HCO
3
, dan Termal. Jurnal Reaktor. UNDIP.
Rahim, M. and Indriyani, O., 2010. Pembuatan Karbon
Aktif dari Batubara Peringkat Rendah. Jurnal
Teknologi Media Perspektif. Politeknik Negeri
Samarinda.
Shawabkeh, R.A., Al-Harthi and Al-Ghamdi, 2014. The
Synthesis and Characterization of Microporous,
High
Surface Area Activated Carbon from Palm Seeds.
Energy Sources, Part A. 36:93–103.
Speight, J.G., 1994. The Chemistry and Technology of
Coal. Marcel Dekker. Inc. New York.
Speight, J. G., 2013a. Coal-Fired Power Generation
Handbook. Beverly, USA. Scrivener Publishing.
Chemical Activation of Lignite by using a Combination of H3PO4-NaHCO3
185
