
Monte Carlo Methods for Assessment of the Mean Glandular Dose in
Mammography: Simulations in Homogeneous Phantoms
R. M. Tucciariello
1
, P. Barca
1
, D. Caramella
4
, R. Lamastra
1
, C. Traino
2
and M. E. Fantacci
1,3
1
Department of Physics, University of Pisa, Pisa, Italy
2
Unit of Medical Physics, Pisa University Hospital “Azienda Ospedaliero-Universitaria Pisana”, Pisa, Italy
3
INFN, Pisa Section, Pisa, Italy
4
Department of Radiology, Pisa University Hospital “Azienda Ospedaliero-Universitaria Pisana”, Pisa, Italy
c.traino@ao-pisa.toscana.it, maria.evelina.fantacci@unipi.it
Keywords: Digital Mammography, Monte Carlo Simulations, Dosimetry, Glandular Dose, Air Kerma, Breast Imaging,
Skin Model, GEANT4, RADIOMA.
Abstract: The rationale of this study is to perform a personalized dosimetry in digital mammography, using Monte Carlo
simulations. We developed a GEANT4-based application that reproduces mammographic investigations
editable in different setups and conditions. Mean Glandular Dose (MGD) is estimated for different
compressed breast sizes and compositions. Breast compositions are obtained with homogeneous mixture of
glandular and adipose tissues. The simulated setup reproduces the Hologic Selenia® Dimensions®
Mammography System and the TASMIP
M
tool for deriving the photon fluence from the X-ray source has
been employed. The influence of different skin models is also investigated, deriving the mean glandular dose
in the breast using adipose tissue for different skin thicknesses, from 2 mm to 5 mm, and a dedicated
composition found in literature with the specific thickness of 1.45 mm. We denoted different photon shielding
properties on the MGD values.
1 INTRODUCTION
In European women, breast cancer is the leading
cause of cancer death, causing one in six of all deaths
from cancers in women. Screening mammography is
a low-dose X-ray examination used to detect breast
cancer, even at an early stage, when that cancer is too
small to be felt as a lump. Digital Mammography
(DM) represents the principal technique used to
reduce this mortality rate and is recommended in
women between 50 and 75. Since ionizing radiation
is used in X-ray mammography investigations, there
is a risk of contracting carcinogenesis associated with
the absorption of X-ray in the mammary gland, which
is considered to be the most radiosensitive tissue at
risk.
A DM investigation is made by compressing the
patient breast with a compression paddle and
acquiring two digital images per breast, a cranio-
caudal and a mediolateral oblique view, with a
polychromatic X-ray source. The Mean Glandular
Dose (MGD) is used for the evaluation of radio-
induced cancer risk and, in principle, this value must
be as low as possible, in agreement with the
investigation image quality. Furthermore, patients
have different compressed breast sizes and
percentage of gland tissue, involving different MGD
values associated to the investigations. Moreover,
skin thickness and different mammography units also
have important effects on radiation dose. Thus,
accurate dosimetry is an important goal to achieve in
X-ray breast imaging.
Dose in the gland tissue can’t be measured
directly, but the use of Monte Carlo (MC) simulations
provides a valuable support. In MC simulations
different variables can be investigated and some
assumptions must be taken into consideration to
estimate the dose delivered to the gland. It is
necessary to create a personalized dosimetry able to
evaluate glandular dose for different anatomical
conditions and commercial mammography units.
The rationale of our study is to perform a
personalized dosimetry in mammography, in which
the MC simulations get closer to the real situations.
Using a GEANT4 based code (https://
geant4.web.cern.ch/), which is an object-oriented
242
Tucciariello, R., Barca, P., Caramella, D., Lamastra, R., Traino, C. and Fantacci, M.
Monte Carlo Methods for Assessment of the Mean Glandular Dose in Mammography: Simulations in Homogeneous Phantoms.
DOI: 10.5220/0007482202420249
In Proceedings of the 12th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2019), pages 242-249
ISBN: 978-989-758-353-7
Copyright
c
2019 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

C++ toolkit for the simulation of the passage of
particles through matter. Its areas of application
include high energy, nuclear and accelerator physics,
as well as studies in medical and space science. This
code has been developed at CERN. We developed a
GEANT4-based application, that reproduces
mammographic investigations, editable in different
setups and breast anatomies.
This study is part of the "RADIOMA" project
(RADiazioni IOnizzanti in MAmmografia, ionizing
radiations in mammography) and must be considered
as a preliminary work.
2 MATERIALS AND METHODS
2.1 State of the Art
Monte Carlo methods are computational methods
based on random sampling to obtain numerical
results; multiple possible realizations of the
phenomenon under examination are calculated, with
the weight of the probability of such occurrence. The
rationale is to run a high number of replications; the
greater the number of the events (photons from the X-
ray source in this case), the greater the accuracy of the
simulation.
When MC simulations are adopted for research on
dosimetry, some model assumptions must be
followed, like breast shape, skin model, adopted
materials, glandular tissue percentage, X-ray
polychromatic source etc… Thus, these assumptions
can seriously affect dosimetry values. For concise
reference henceforward, the breast composition is
referred to in terms of the glandular percentage by
weight.
Dose to the breast starts in general from incident
Air Kerma (K
air
), air dose measurement, converted by
dedicated coefficients to obtain a reference value of
dose.
The European mammography dosimetry protocol
employs the model proposed by Dance (Dance,
1990). In the MC simulations the breast is modelled
as a semi-cylinder, with radius of 80 mm and variable
height between 20 and 110 mm, with inside a
homogeneous compound of adipose and glandular
tissues surrounded by a 5 mm thick skin made by
adipose tissue. The conversion factors calculated by
the author are for a breast model of 50% glandularity
and are tabulated as a function of the breast thickness
and the Half Value Layer (HVL) of the X-ray beam.
The formalism used to calculate the Average
Glandular Dose, AGD, is
AGD = K
air
g c s ,
(1)
where K
air
is the incident air kerma without
backscatter at the upper surface of the breast, is the
conversion factor for a 50% glandularity breast at the
specified HVL, and and factors correct for breast
composition and X-ray spectrum choice respectively.
The US protocol follows the Wu’s method (Wu,
1991; Wu, 1994), in which the breast shape is a semi-
cylinder but with a semi-elliptical cross-section. The
breast model has a 5 mm thick skin layer of adipose
tissue (indeed, skin thickness was considered to be 4
mm until the new 2016 ACR Digital mammography
quality control manual), while the inner part is a
homogeneous mixture of adipose and glandular
tissues; the reference relative amounts of glandular
tissue are 0%, 50% and 100%. The Mean Glandular
Dose, MGD, is obtained multiplying K
air
by a factor
denoted as normalized glandular dose ()
MGD = K
air
DgN .
(2)
Using Monte Carlo simulations, the authors
tabulated DgN values for breasts as defined above
and for X-ray spectra derived from a molybdenum
target and molybdenum filter, varying the phantom
breast thickness from 3 to 8 cm.
The maximum dose limits for the “standard
breast” (Yaffe, 2009) are, in digital mammography,
per view, 2.5 mGy in EU protocols and 3 mGy in US
protocols.
In the last years, a trend has emerged to extend
and perform protocols and research groups are
proposing their models and methods (Sechopoulos,
2012; Traino, 2017; Sottocornola, 2018). The
rationale of our study is to perform a personalized
dosimetry in mammography, in which the MC
simulations get closer to the real situations,
simulating real mammography investigations
executed with the Hologic Selenia® Dimensions®
mammography system. For personalized dosimetry
we mean the objective to evaluate both different
breast anatomies and commercial mammography
systems, with other anode/filter combinations, that of
course traduces in different glandular dose values.
The GEANT4-based application developed by
our group reproduces mammographic investigations
in different setups and breast anatomies. The code
provides, in the same run, simulation of mean
glandular dose and incident air kerma at the upper
surface of the breast (backscatter photons are
excluded from the computation). This led to a
reduction in the simulation times. Using an Intel
Core
TM
i7 8700 CPU @ 4.30 GHz (12 threads
Monte Carlo Methods for Assessment of the Mean Glandular Dose in Mammography: Simulations in Homogeneous Phantoms
243
available), 32GB of RAM, multithreading mode
performs 10
8
events in approximately 10 minutes.
2.2 Code Validation and
Characteristics
The code was validated according to the prescription
of The American Association of Physicists in
Medicine, AAPM Task Group 195 (Case III, for
mammography purposes). The code showed
discrepancies from the reference data of 0.6% with
both monoenergetic and polyenergetic X-ray beams
in MGD scoring, which is computed by
(3)
where
is the G-factor introduced by (Boone,
1999) evaluated for the energy of the jth interacting
photon,
the energy deposition of this photon and
the glandular mass. For Air Kerma scoring,
results are in agreement with data in literature (Sarno,
2017), using
(4)
where
is the energy of the ith incident photon
passes through the scoring surface S,
is
the air mass energy absorption coefficient at the
energy
(Hubbel, 1995). K
air
computation let to
obtain estimates of dose conversion coefficients to be
used or compared with data in literature (Boone,
2002; Nosratieh, 2015).
This kind of validation is useful due to the
opportunity to compare results with those given by
other groups, that use different MC codes
(Gholamkar, 2016) but the same methodology used in
the protocols.
Furthermore, other research groups propose their
physical phantom models, create to validate their MC
code, using, for example, TLD dosimeters (Wang,
2017; Nigapruke, 2010).
2.2.1 Implemented Geometry
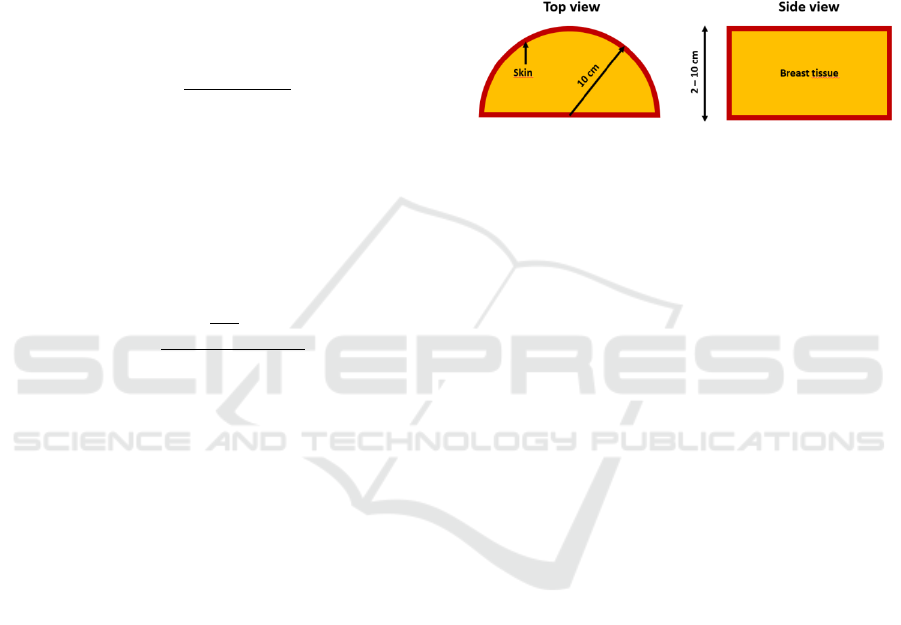
A semi-cylinder was used to simulate a compressed
breast, with a radius of 10 cm, variable heights that
correspond to the different compressed breast
thicknesses, from 2 cm to 10 cm in 1 cm increment,
and 5 breast compositions, 0% 12.5% 25% 50% and
100% glandular fractions. Different glandular
fractions are obtained mixing properly adipose and
glandular tissue, from data provided by (Boone,
1999), in order to obtain homogeneous mixtures with
desired glandularities (Dance, 2016; Sarno 2018).
A skin thickness of 5 mm of adipose tissue was
introduced to the model and two polycarbonate
compression paddles, upper (2.8 mm thick) and lower
(4.1 mm thick).
As prescribed by the AAPM TG195 protocol, a
box made by water is used to consider the scattered
radiation from the patient body (Figure 2).
Figure 1: Breast model adopted. The semi-cylinder radius
is fixed to 10 cm, the range thickness is 2-10 cm while the
skin is investigated changing its thickness.
2.2.2 Polychromatic Source
The X-ray source is positioned following the Hologic
Selenia® Dimensions® mammography system
geometry. The X-ray beam simulates a craniocaudal
view and it is produced by a focal spot of 0.3 mm
2
at
a source-to-image receptor distance (SID) of 70 cm.
Since real mammographic exams involve a
polychromatic X-ray beam source, in order to obtain
data that are comparable with real investigations, in
the MC code a polychromatic spectra can be set as a
macro file, in which every energy bin is weighted
with its relative photon fluence; this weight is used in
the computation as a statistical probability to produce
an incidence photon in its energy bin. Thus, we use
TASMIP
M
, algorithm for tungsten anode material,
provided by (Boone, 1997), to produce photon
fluences referring to the Selenia® Dimensions®
system, to assess glandular dose in digital phantoms.
The algorithm provides spectra for the voltage
applied ranging from 18 to 40 kV; spectral
information is released at 500 eV intervals starting
from 5.5 to 40 keV, with energy bins centered in 5.5,
6.0, 6.5…
BIOINFORMATICS 2019 - 10th International Conference on Bioinformatics Models, Methods and Algorithms
244
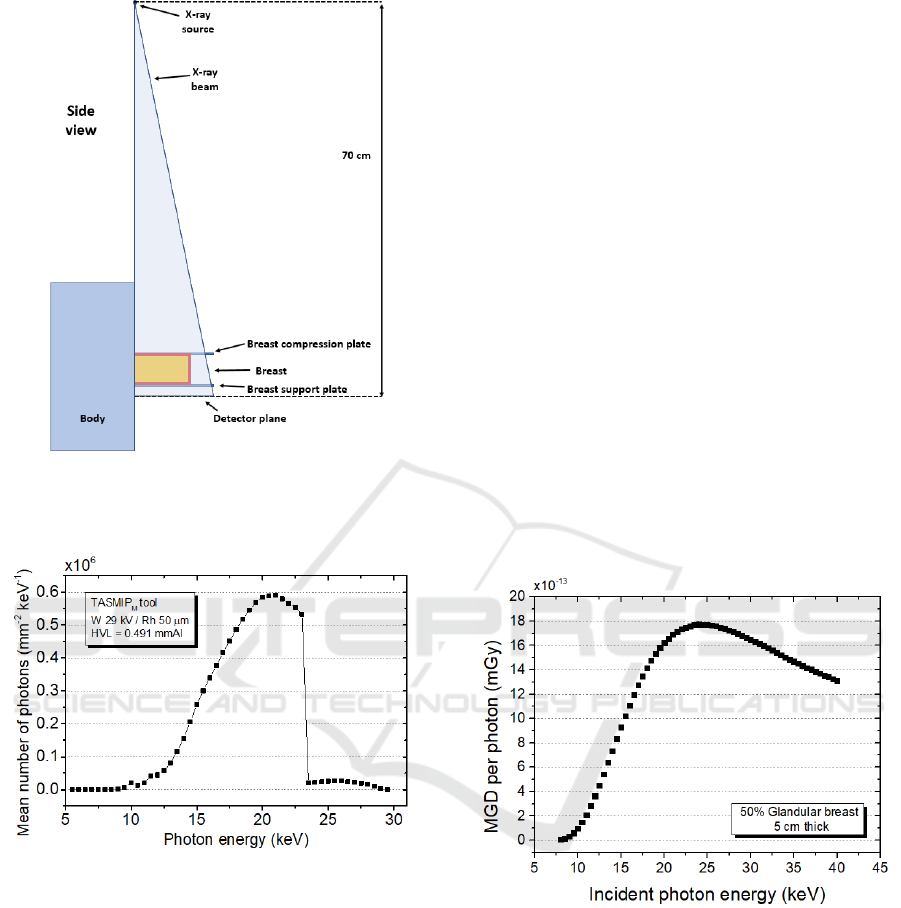
Figure 2: Simulation geometry adopted. Source to detector
plane distance is set to the SID of the Hologic Selenia®
Dimensions® system.
Figure 3: Example of spectra obtained using TASMIP
M
algorithm, in W/Rh anode/filter combination @ 29 kV.
Spectra is normalized to unit Air Kerma.
2.2.3 Physics List
According to the prescriptions provided by the report
of AAPM Task Group 195, the “Option4”
PhysicsList was used in GEANT4, for the
constructors and instances that consider the physics
processes; this model is designed for any application
requiring high accuracy of electrons and it uses the
most accurate standard low-energy models. The
production threshold (“range cut”) fixed for the
secondary particles is expressed in terms of the
distance travelled by the particles in the medium (skin
or breast tissue), converted by GEANT4 in terms of
energy; e.g. the range cuts of 1 mm for photons and 1
µm for electrons correspond respectively to about
2.55 keV and 0.99 keV in 25% glandular breast
tissue. For energies involved in these cases Rayleigh,
photoelectric, Compton and bremsstrahlung are
simulated.
3 RESULTS
With the simulation setup described in the previous
section, MGD and K
air
were obtained. For
monoenergetic beams, we decided to focus in the
energy spectrum between 8 keV and 40 keV, with 0.5
keV step, range in which photons are produced by
mammography X-ray tubes. Figure 4 shows the MGD
per generated photon, for a 5 cm thick breast with
50% glandular fraction and a 5 mm thick skin made
by adipose tissue. For monochromatic purposes we
used 10
7
simulated events. The simulation model
considers the energy deposited in the breast tissue,
excluding skin; thus, at lower photon energies, the
skin “shields” the breast tissue and the delivered dose
is low.
Figure 4: Dose per photon delivered to glandular tissue to a
50% glandular breast and 5 cm thick, due to both the
primary and the secondary radiation. Each point in the
graph represents one simulation run with a monochromatic
beam with 10
7
simulated events.
At increasing photon energies, X-ray beam
penetrates the skin layer and deposits dose, up to a
maximum of about 23 keV; then, the total dose to the
glandular breast reduces, due to the decreasing
energy-absorption coefficient.
Of course, we concentred our efforts on
polychromatic spectra; for polyenergetic beams we
simulated 10
8
primary events. In Figure 5 it is
represented the mean glandular dose versus
Monte Carlo Methods for Assessment of the Mean Glandular Dose in Mammography: Simulations in Homogeneous Phantoms
245
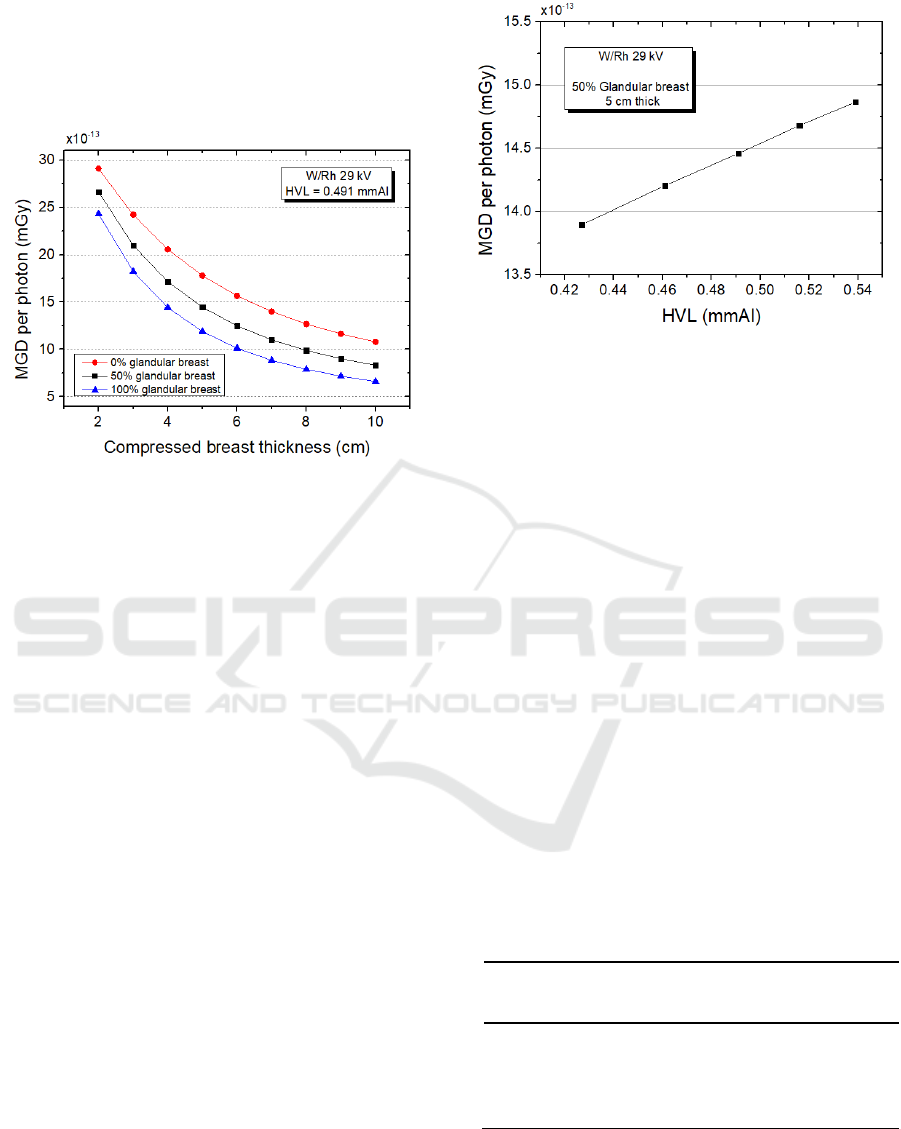
compressed breast thickness for the same X-ray beam
source and 0%, 50% and 100% glandular tissues.
MGD values decrease while increasing compressed
breast thickness and/or glandular fraction, due to a
major glandular mass in the equation (3).
Figure 5: MGD per photon vs. compressed breast thickness
for different glandularities in W/Rh configuration @ 29 kV.
Each point on the graph refers to a single simulation with
10
8
events.
Furthermore, beam quality, in terms of half value
layer (HVL, units of mm Al) has to be considered
(Sobol, 1996). Spectra provided by algorithms are not
correct at all, because of the uncertainty of the filter
thickness (usually estimated by the manufacturer on
about 10%) and, of course, due to the algorithm
adopted approximations. This may lead to either an
overestimation, or underestimation, of low energy, or
high energy, of photons, and a consequent mismatch
on dose assessment. To avoid this circumstance a
beam quality estimate has to be performed.
Once one knows the experimental value, the
rationale is to try to reach the correct HVL value on
the algorithm varying the filter thickness. Figure 6
shows the dependence of MGD from the half value
layer of the radiation; a harder beam (i.e. a relatively
major number of photons with higher energy) delivers
more glandular dose to the breast.
Since mammary gland is considered to be the
tissue at risk, one has to consider skin as a shielding
tissue. The greater the thickness, the greater the
shielding. Unfortunately, in literature there are
bucking studies about skin thickness and
composition. The EU and US protocols used until the
2016 different skin thickness, of 5 mm and 4 mm
respectively, made by adipose tissue. The reason is
that skin is composed by three parts, starting from
outside to inside by epidermis, dermis and
hypodermis. It is not possible to differentiate them in
Figure 6: MGD vs. HVL. Different HVL values are
obtained varying the Rh filter thickness from 40 to 60 µm
with 5 µm step.
a clear manner, because thickness and distribution are
very variable, but it is evident that dermis and
hypodermis are mainly composed by adipose tissue.
Nevertheless, only the epidermis, the outer layer, is
evident from breast Computed Tomography (bCT)
images (Huang, 2008), which has a higher density
from adipose tissue. This involves different skin
attenuation and shielding. Because of its obvious
presence in the TC slices, other research groups
(Sarno, 2017; Massera, 2018) tend to consider the
epidermis layer in their respective studies, whose
average thickness is 1.45 ± 0.30.
We wanted to investigate the effect of various skin
models on MGD values, changing thickness and
compositions, simulating, as previously,
monoenergetic and polyenergetic beams. In Table 1
five types of skin model adopted are reported, whose
surround the same 50% phantom glandularity to form
five different 5 cm thick digital phantoms.
Table 1: Skin models adopted. These models are associated
to 50% glandular and 5 cm thick digital breast to form five
different phantoms.
Skin
model
Skin
thickness
Skin
composition
Density
[g/cm
3
]
#1
1.45 mm
Skin (Boone, 1999)
1.09
#2
2 mm
Adipose tissue
0.93
#3
3 mm
Adipose tissue
0.93
#4
4 mm
Adipose tissue
0.93
#5
5 mm
Adipose tissue
0.93
Obviously, a thicker skin traduces in a major
photon shielding of the mammary gland, but models
#1 and #5 (which is adopted by EU protocol), due to
the different skin compositions and thicknesses,
BIOINFORMATICS 2019 - 10th International Conference on Bioinformatics Models, Methods and Algorithms
246
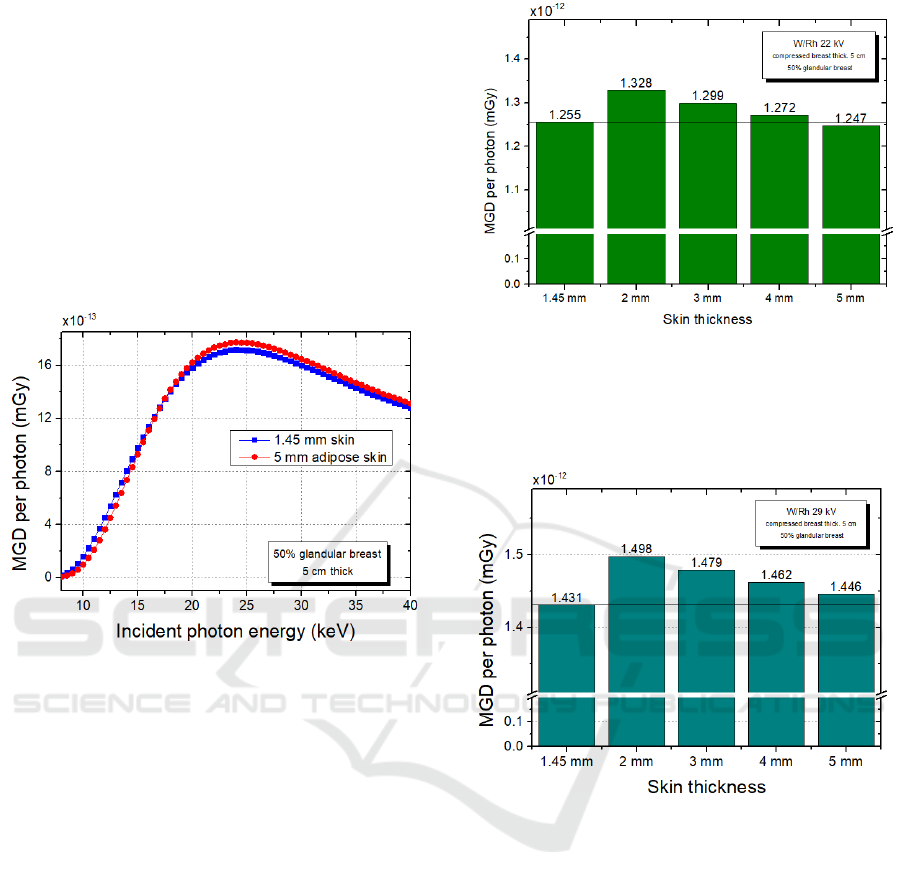
deserve a brief comparison. In Figure 7 different
shielding properties are denoted. For low energies,
the first breast model is less shielded by its (thin) skin,
showing slightly higher MGD values; a reverse
situation appears for higher energies; this may be
attributed to the property of the thicker skin to
“become a secondary radiation source” at increasing
energies, because of the probability of the Compton
effect (in the skin) increase at the expense of the
photoelectric effect (Sarno, 2017). These results have
been also found by Massera et al. (Massera, 2018),
who used PENELOPE, another Monte Carlo code, to
produce MGD values for monochromatic beams.
Figure 7: MGD vs. photon energy for monoenergetic beams
ranging from 8 to 40 keV, for phantoms #1 and #5.
In order to better outline this behaviour with
polychromatic beams, we reproduce two W/Rh
kilovoltage configurations, 22 kV and 29 kV. It is
evident in figures 8 and 9 that, for the same skin
composition, the thicker is the skin, the lower is the
dose to the gland; nevertheless, if we consider the #1
and the #5 skin models, we see that the last shields
more the breast tissue at @ 22 kV (Figure 8), but less
@ 29 kV (Figure 9). This result confirms the previous
statement in the monoenergetic investigation.
4 DISCUSSION
The aim of this project is to obtain a personalized and
accurate dosimetry for X-ray digital mammography,
for different breasts sizes and composition and
commercial mammography units commonly used.
Figure 8: Dose delivered to glandular tissue versus different
skin thicknesses and compositions. 1.45 mm thick skin has
a different elemental composition (Boone, 1999), while
from 2 mm to 5 mm skin is made by adipose tissue.
Simulations refer to a low-energy examination.
Figure 9: Dose delivered to glandular tissue versus different
skin thicknesses and compositions. Simulations refer to a
higher energy examination.
We developed, and opportunely validated, a
GEANT4 Monte Carlo code for dosimetry in
mammography; adopting some geometry
assumptions and an external algorithm (Boone, 1997;
Hernandez, 2017) for deriving spectral information
for mammography X-ray tubes, the code is able to
replicate different mammography setups for different
geometries and conditions, and different anatomical
women breasts.
In order to assess the Mean Glandular Dose in
digital phantoms for typical mammography
investigations, we used the TASMIP
M
tool to
reproduce photon fluences referring to the Hologic
Selenia® Dimensions® system, adopted in the
Department of Radiology, University Hospital
“Azienda Ospedaliero-Universitaria Pisana”, Pisa.
Monte Carlo Methods for Assessment of the Mean Glandular Dose in Mammography: Simulations in Homogeneous Phantoms
247

We derived the dependencies of the Mean
Glandular Dose changing breast anatomy and X-ray
beam; MGD decrease with both the increase of
compressed breast thickness and glandular
percentage. Important dependency is represented by
the HVL value (radiation beam quality), which can
lead to an overestimation of the glandular dose in case
of “harder” spectra. The rationale is to know the HVL
experimental value and to find the correct MGD value
referring to the specific radiation.
An important variable not yet permanently
defined in literature is the assessment of skin
thickness and composition. EU and US protocols
used, until 2016, respectively 5 mm and 4 mm thick
skin (now US protocol employs the 5 mm thick skin),
made by adipose tissue but, Huang et al. (Huang,
2008) found a different skin thickness in breast CT
investigations. A comparison between digital breast
phantoms with different skin showed of course
different MGD values. At low-energy investigations
skin 1.45 mm thick “shields” less glandular tissue,
respect to the 5 mm adipose skin, while for higher
energies shields more. This may be attributed to the
property of the thicker skin to “become a secondary
radiation source” at increasing energies, because of
the probability of the Compton effect (in the skin)
increases at the expense of the photoelectric effect
(Sarno, 2017). The choice of an appropriate model of
a digital breast phantom can be a critical aspect and
we reserve the right to continue investigating it.
In the last years, Digital Breast Tomosynthesis
(DBT) is spreading in clinics and represents an
evolution of mammography; this technique let the X-
ray tube to move in an arc over the compressed breast,
acquiring multiple images from different angles.
Images are then reconstructed by a computer forming
three-dimensional images. 3D techniques minimize
tissue overlaps that can hide cancers above the normal
overlapping.
We will improve our MC code implementing the
tomosynthesis set-up for dosimetry purposes.
To achieve the experimental verification of the
MC results, and improve the personalized dosimetry,
our efforts are focused on the creation of physical
phantoms with similar properties of the real breast,
like, of course geometry, but primarily X-ray
attenuation.
ACKNOWLEDGEMENTS
The presented work is part of the RADIOMA project
which is partially funded by "Fondazione Pisa",
Technological and Scientific Research Sector, Via
Pietro Toselli 29, Pisa (Italy). The authors would like
to thank Fondazione Pisa for giving the opportunity
to start this study.
REFERENCES
AAPM REPORT NO. 195. 2015. Monte Carlo Reference
Data Sets for Imaging Research.
Agostinelli, S. et al., 2003. GEANT4 - a simulation toolkit.
Nuclear Instruments and Methods in Physics Research
A 506 (2003) 250–303.
Boone, J.M., Fewell, T.R., Jennings, R.J., 1997.
Molybdenum, rhodium, and tungsten anode spectral
models using interpolating polynomials with
application to mammography. Med Phys. 1997
Dec;24(12):1863-74.
Boone, J.M., 1999. Glandular Breast Dose for
Monoenergetic and High-Energy X-ray Beams: Monte
Carlo Assessment. Radiology. 1999 Oct;213(1):23-37.
Boone, J.M., 2002. Normalized glandular dose (DgN)
coefficients for arbitrary x-ray spectra in
mammography: Computer-fit values of Monte Carlo
derived data. Med Phys. 2002 May;29(5):869-75.
Dance, D.R., 1990. Monte Carlo Calculation of conversion
factor for the estimation of mean glandular breast dose.
Phys Med Biol. 1990 Sep;35(9):1211-9.
Dance, D.R., Sechopoulos, I., 2016. Dosimetry in x-ray
based breast imaging. Phys Med Biol. 2016 Oct
7;61(19):R271-R304.
Gholamkar, L., Mowlavi, L.,A., Sadeghi, M., Athari, M.,
2016. Assessment of Mean Glandular Dose in
Mammography System with Different Anode-Filter
Combinations Using MCNP Code. Iran J Radiol. 2016
October; 13(4):e36484.
Hernandez, A.M., Seibert, J.A., Nosratieh. A,, Boone, J.M.,
2017. Generation and analysis of clinically relevant
breast imaging x-ray spectra. Med Phys. 2017
Jun;44(6):2148-2160.
Huang, S., Boone, J.M., Yang, K., Kwan, A.L.C., Packard,
N.J., 2008. The effect of skin thickness determined
using breast CT on mammographic dosimetry. Med
Phys. 2008 Apr;35(4):1199-206.
Hubbell, J.H., Seltzer, S.M., 1995. Tables of X-Ray Mass
Attenuation Coefficients and Mass Energy-Absorption
Coefficients from 1 keV to 20 MeV for Elements Z = 1
to 92 and 48 Additional Substances of Dosimetric
Interest. NISTIR 5632.
Massera, R.T., Tomal, A., 2018. Skin models and their
impact on mean glandular dose in mammography.
Physica Medica Volume 51, July 2018, Pages 38-47.
Nigapruke, K., Puwanich, P., Phaisangittisakul, N.,
Youngdee, W., 2010. Monte Carlo Simulation of
Average Glandular Dose and an Investigation of
Influencing Factors. Journal of Radiation Research,
2010 51(4), 441–448.
Nosratieh, A., Hernandez, A,. Shen, S.Z., Yaffe, M.J.,
Seibert, J.A., Boone, J.M., 2015. Mean glandular dose
coefficients (DgN) for x-ray spectra used in
BIOINFORMATICS 2019 - 10th International Conference on Bioinformatics Models, Methods and Algorithms
248

contemporary breast imaging systems. Phys. Med. Biol.
60 7179.
Sarno, A., Mettivier, G., Russo, P., 2017. Air kerma
calculation in Monte Carlo simulations for deriving
normalized glandular dose coefficients in
mammography. Phys. Med. Biol. 62 (2017) N337–N349.
Sarno, A., Mettivier, G., Di Lillo, F., Russo, P., 2017. A
Monte Carlo study of monoenergetic and polyenergetic
normalized glandular dose (DgN) coefficients in
mammography. Phys. Med. Biol. 62 (2017) 306–325.
Sarno, A., Mettivier, G., Di Lillo, F., Bliznakova, K.,
Sechopoulos, I., Russo, P., 2018. Homogeneous vs.
patient specific breast models for Monte Carlo
evaluation of mean glandular dose in mammography.
Physica Medica, 2018 Jul; 51, 56–63.
Sechopoulos, I., Bliznakova, K., Qin, X., Fei, B., Feng,
S.S.J, 2012. Characterization of the homogeneous
tissue mixture approximation in breast imaging
dosimetry. Med Phys. 2012 Aug;39(8):5050-5059.
Sobol, W.T., Wu, X., 1996. Parametrization of
mammography normalized average glandular dose
tables. Med Phys. 1997 Apr;24(4):547-54.
Sottocornola C., Traino A.C., Barca P., Aringhieri G.,
Marini C., Retico A., Caramella D., Fantacci M.E.,
2018. Evaluation of Dosimetric Properties in Full Field
Digital Mammography (FFDM) Development of a New
Dose Index. Proceedings of the 11th International Joint
Conference on Biomedical Engineering Systems and
Technologies (BIOSTEC 2018) - Volume 1:
BIODEVICES, 212-217.
Traino A.C., Sottocornola C., Barca P., Marini C.,
Aringhieri G, Caramella D, Fantacci ME. 2017.
Average absorbed breast dose in mammography: a new
possible dose index matching the requirements of the
European Directive 2013/59/EURATOM. European
Radiology Experimental 1:28.
Wang, W., Qiu, R., Ren, L., Liu, H., Wu, Z., Li, C., Li, J.,
2017. Validation of Monte Carlo simulation of
mammography with TLD measurement and depth dose
calculation with a detailed breast model. EPJ Web of
Conferences, 2017 153, 04017.
Wu, X., Barnes, G.T., Tucker, D.M., 1991. Spectral
dependence of glandular tissue in screen-film
mammography. Radiology. 1991 Apr;179(1):143-8.
Wu, X. Gingold, E.L., Barnes, G.T., Tucker, D.M., 1994.
Normalized average glandular dose in molybdenum
target-rhodium filter and rhodium target-rhodium filter
mammography. Radiology. 1994 Oct;193(1):83-9.
Yaffe, M.J., Boone, J.M., Packard, N., Alonzo-Proulx, O.,
Huang, S.Y., Peressotti, C.L., Al-Mayah, A., Brock, K.,
2009. The myth of 50-50 breast. Med Phys. 2009
Dec;36(12):5437-43.
Monte Carlo Methods for Assessment of the Mean Glandular Dose in Mammography: Simulations in Homogeneous Phantoms
249
