
Cell Deformability Studies for Clinical Diagnostics: Tests with Blood
Analogue Fluids using a Drop based Microfluidic Device
A. S. Moita
1
, C. Caldeira
1
, F. Jacinto
1
, R. Lima
2,3
, E. J. Vega
4
and A. L. N. Moreira
1
1
IN
+
Center for Innovation, Technology and Policy Research, Instituto Superior Técnico, Universidade de Lisboa,
Av. Rovisco Pais, 1049-001, Lisboa, Portugal
2
CEFT, Faculdade de Engenharia da Universidade do Porto (FEUP), R. Dr. Roberto Frias, 4200-465, Porto, Portugal
3
Metrics, Mechanical Engineering Department, University of Minho, Campus de Azurém, 4800-058, Guimarães, Portugal
4
Área de Mecánica de Fluidos, Dpto. de Ingeniería Mecánica, Energética y de los Materiales, Escuela de Ingenierias
Industriales, Universidade de Extremadura, Campus Universitario, Av. de Elvas, s/n, 06006, Badajoz, Spain
rl@dem.uminho.pt, ejvega@unex.es
Keywords: Lab-on-a-chip, Droplet based Microfluidics, Clinical Diagnostics, Cell Deformability, Bioanalogue Fluid.
Abstract: The present paper addresses the final tests (concerning the transport section) of a microfluidic device to be
used in cancer diagnostics, based on the mechanical properties of the cells and particularly on deformability.
Following the previous work, which established the materials to be used, according to the wetting properties
and their influence on the dynamic response of the droplets (which are electrostatically actuated) this paper
presents the final simulations to optimize the thickness and material of the dielectric coating, always as a
function of the dynamic response of the droplets. Then, to avoid contamination issues, a number of analogue
fluids are proposed, in a new approach, which are characterized and tested in the second part of the work.
Regarding the characterization of these new fluids, preliminary results suggest a great potential of a
surfactant solution to be used as an analogue. The addition of the surfactant results in the formation of semi-
rigid particles with a size distribution and deformation characteristics compatible with those of the
biosamples to be studied. The surfactant solution also shows a swift response to electrostatic actuation.
1 INTRODUCTION
Lab-on-chip devices are pointed as strongly
effective tools to perform a number of complex
sample manipulation operations, biochemical
analysis and immunoassay tests (e.g. Takahashi et
al., 2004, Gossett et al., 2010, Shields et al., 2015,
Chim, 2015). Besides allowing a significant
reduction of the samples and of the reagents as well
as a better control of the reactions, due to the small
characteristic time and length scales, the
microfluidic devices offer a significant energy
reduction, are easy to use and portable. Furthermore,
errors and contamination issues are precluded, since
the manual handling is marginal (Lin et al., 2010).
Sample transport in continuous medium using
microchannels is probably the most common
microfluidic design, but addresses a number of
inconveniencies such as the need for auxiliary
systems, which are still very ineffective from the
energetic point of view, clogging, difficulties in
accessing the samples, among others (Geng et al.,
2017). In this context, droplet-based microfluidics is
considered by several authors as an effective
alternative (e.g. Pollack et al., 2011, Dance, 2017).
Droplet handling can be performed using different
kinds of external actuation, (e.g. Zeggari et al.,
2014) although electrowetting is amongst the most
popular and well grounded. However, although
theoretical background on electrowetting is already
well recognized, as revised for instance in Mugele
and Baret (2005) and more recently in Nelson et al.
(2012) the details required for an effective design
and assembly of the chips is scarcely reported in the
literature (e.g. Li et al., 2012). Hence, optimization
of the chip design requires a deep knowledge on the
wetting properties of the materials used (e.g. Chim,
2015, Vieira et al., 2017), as well as an accurate
description of biosamples fluid dynamics and respon-
se under external actuation (e.g. Moita et al., 2016).
Microfluidic devices are also suitable to take
advantage of particular properties of the biosamples,
towards the development of label free diagnostic
approaches. In this context, mechanical properties of
Moita, A., Caldeira, C., Jacinto, F., Lima, R., Vega, E. and Moreira, A.
Cell Deformability Studies for Clinical Diagnostics: Tests with Blood Analogue Fluids using a Drop based Microfluidic Device.
DOI: 10.5220/0007578100990107
In Proceedings of the 12th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2019), pages 99-107
ISBN: 978-989-758-353-7
Copyright
c
2019 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
99

the cells and particularly their deformability are a
hallmark to identify several diseases, such as cancer.
Characterizing cancer cell deformation can provide
useful information on the process of metastasis,
particularly for large deformability regimes, which
are rarely described in the literature (Hu et al.,
2018). Furthermore, a few authors have drafted
preliminary correlations between the deformability
ratio of the cells and the stage of malignancy (e.g.
Tse et al., 2013). At the microscale, it is also
expected that droplet dynamics can be sensitive to
the rheological variations caused by different
degrees of cell stiffness/deformability, so droplet
dynamics could be eventually correlated with cell
stiffness for early stage cancer diagnostics (e.g.
Vieira et al., 2017).
Following our previous work (Jacinto et al.,
2018), the present paper focuses on the development
of a droplet based microfluidic system, intended to
be used in clinical diagnostics (lung cancer) based
on cell deformability and its possible correlation
with the fluid dynamics of the droplets used for the
sample handling. The paper is organized in two main
sections: the first summarizes the process of design
and optimization of the lab-on-chip device, whose
configuration (electrodes dimensioning and
positioning, materials and wetting properties) were
optimized based on the dynamic behavior
(spreading, contact diameter and velocity) of the
biosample droplets. Then, following an original
approach, a brief description is made on the
procedure which will be used to infer on a possible
correlation between the cells deformability and
different stages of malignancy, following the work
of Tse et al. (2013). It is worth mentioning that at
this stage of the work, analogue fluids are proposed
to be used in the preliminary assays, to overcome
contamination issues, among others. Within this
scope, different approaches are considered in the
present work, to devise the analogue fluids, namely,
using xanthan gum solutions and suspensions of
polymers and surfactants solved on water, giving
rise to small semi-rigid particles. The main physical
properties of the fluids are accessed and they are
characterized in terms of the resulting particle size
distribution, evaluated based on Laser Scanning
Fluorescence Confocal Microscopy. The size and
deformability of the resulting particles is then tested
to check on their feasibility to mimic cell
deformability in the context of the current main goal
of this work (malignancy diagnostics). The dynamic
response of the analogue fluids is also tested to
check on the feasibility of their handling in the
microfluidic device.
2 MATERIALS AND METHODS
2.1 Numerical Method
In our previous work, the materials used to assemble
the microfluidic device were carefully selected,
based on their wetting properties and on an intensive
analysis to minimize adsorption issues, which were
affecting sample handling, besides being an obvious
source of contamination (Vieira, et al., 2017, Moita
et al., 2018). A numerical approach was then
followed to predict the motion of the sample
microdroplets, optimizing the chip configuration to
promote droplet motion (Jacinto et al., 2018).
Following these steps, a final numerical optimization
was performed to check on the correct selection of
the dielectric materials which are used to coat the
electrodes. Their thickness is also optimized, to
maximize the resulting electrostatic force, the
distance travelled by the droplet and the velocity of
the moving droplets. These parameters were
optimized for the lowest possible applied voltage.
The numerical study was performed using
COMSOL Multiphysics 4.3b. The electrostatic forces
actuating on the droplets were determined using
Maxwell stress tensor, integrated on droplets’ surface.
Droplet motion was simulated using an
incompressible formulation of Navier-Stokes
equations for a laminar flow. Phase Field User
Interface was used to track the liquid-air interface.
The complete model formulation together with the
detailed description of the computational domain
and boundary conditions used can be found in
Jacinto et al. (2018).
2.2 Experimental Method
An experimental approach, as proposed in the
present work, was followed to characterize the
analogue fluids and check on their suitability to infer
on possible correlations between the deformability
of the cells and different stages of malignancy. The
analogue fluids were characterized in terms of their
main physico-chemical properties and on the size
distribution of the particles mimicking the cells, as
described in the following sub-sections. The
electrostatic response of the various analogue fluids
tested here was also inferred, using the experimental
arrangement detailed below.
2.2.1 Experimental Arrangement
The dynamic response of the analogue fluids
wasevaluated on a simplified arrangement. In this
BIODEVICES 2019 - 12th International Conference on Biomedical Electronics and Devices
100

arrangement, a 25μm diameter tungsten wire
(Goodfellow Cambridge Ltd), acting as an electrode,
was dipped inside the droplet to be tested. The
counter electrode on which the droplet was
deposited was a copper cylinder with 19mm of
diameter and 20mm height. A 10μm Teflon film
(Goodfellow Cambridge Ltd) was used as the
dielectric layer. As recommended by Restolho et al.
(2009), a very thin film of sodium chloride was
placed between the counter electrode and the
dielectric to avoid the presence of an air gap. Both
electrodes were connected to a Sorensen DCR600-
.75B power supply and DC voltage was applied. The
tests were performed inside a Perspex chamber with
quartz windows to avoid optical distortion, under
continuously controlled temperature and relative
humidity conditions. The chamber was previously
saturated with the working fluid, for each fluid used.
Relative humidity measurements were taken at a
sample rate of 0.5Hz, with an accuracy of 2-5%.
Temperature measurements were taken also at a
sample rate of 0.5Hz, within ±0.5°C accuracy.
Measurements were performed using a DHT 22
Humidity & Temperature Sensor. The temperature
was observed to be constant within T=20±3ºC and
relative humidity was kept constant between 75%
and 78%. This entire set-up was directly mounted on
an optical tensiometer THETA, from Attention.
Using the sessile drop method, the equilibrium
contact angle (the angle formed in equilibrium,
between the surface line and a tangent line touching
droplet edge) was evaluated as a function of the
applied voltage, for a range between 0-230V in 25V
increments. The final curves presented and discussed
here were averaged from at least six assays, obtained
under similar conditions. Droplet volume was kept
constant and equal to 3μl.
The detailed description of the set-up (with the
appropriate schematics) and experimental procedu-
res which were used here to access the dynamic
response of the analogue fluids under electrostatic
actuation can be found in Moita et al. (2016).
2.2.2 Preparation of the Analogue Fluids
and Characterization of their
Physico-chemical Properties
Analogue fluids were prepared following three
different strategies, namely using xanthan gum,
using a suspension of polymeric particles in water
DD and mixing water DD with a small quantity of
surfactant which results in the formation of semi-
rigid surfactant particles.
The xanthan gum solutions were prepared with
the concentrations of 0.05wt% and 0.35wt%. As
these solutions have a shear thinning behaviour, the
viscosity vs shear rate curves were fitted using the
Cross model (Cross, 1965), following the procedure
described in Moita et al. (2015). Rheological data
were measured under controlled temperature
conditions, at ATS RheoSystems (a division of
CANNON® Instruments, Co). The accuracy of the
measurements is within ±5%. The suspensions with
polymeric particles were prepared with PMMA -
Poly(methyl methacrylate) dissolved in water DD
(1wt%). Different concentrations are expected to be
tested in the near future. Seeking at appropriate
characteristic spatial scales that could be used to
mimic the cells, different particle diameters were
tested here, namely 5μm, 10μm and 20μm. Finally,
the third approach consisted in adding a surfactant
Brij40 (a nonionic polyoxyethylene surfactant from
Thermo Fisher) which results in the formation of
semi-rigid micro-particles suspended in the water.
Surface tension
σ
lv
was measured using the
optical tensiometer (THETA from Attention). The
final surface tension values were averaged from 15
measurements taken under controlled temperature
conditions (20±3ºC). Measurements have standard
mean errors always lower than 0.35. Density
ρ
was
measured with a pycnometer for liquids and
concentrations were checked by basic concentration
calculations. The detailed description of the
measurement procedures can be found in Moita et
al. (2018). Table 1 summarizes the main physico-
chemical properties of the various fluids tested here.
It is worth mentioning that varying the diameters
of the PMMA particles did not produce any
significantly quantifiable modification in the density
or in the surface tension of the fluids tested here.
Table 1: Density and surface tension (measured at room
temperature 20±3ºC) of the analogue fluids tested in the
present work.
Solution Density
ρ [kg/m
3
]
Surface
tension
σ
lv
[mN/m]
Xanthan
gum
0.05wt%
997 72.0
Xanthan
gum
0.35wt%
997 72.95
Water DD
+ PMMA
particles
(1wt%)
999 58.65
Water DD
+ Brij40
999 21.10
Cell Deformability Studies for Clinical Diagnostics: Tests with Blood Analogue Fluids using a Drop based Microfluidic Device
101

2.2.3 Characterization of Particles Sizes and
Deformability using Laser Scanning
Fluorescent Confocal Microscopy
Analogue fluids were further characterized in terms
of the size distribution of their particles and of their
deformability capability (to mimic the various
degrees of cell deformability associated to the
different stages of malignancy). The analysis of the
size distribution was performed based on extensive
post-processing of images taken with a Laser
Scanning Confocal Microscope (SP8 from Leica),
using an in-house code developed in MATLAB. The
images were taken using Rhodamine B (Sigma
Aldrich) as fluorophore in a concentration of
3.968x10
-6
g/ml, which does not alter the physico-
chemical properties of the analogue fluids. A laser
with 552 nm wavelength, was used. The power of
the laser was set to 10.50 mW (3.00% of its
maximum power) and the gain of the
photomultiplier was fixed at 550V. These values
were chosen after a sensitivity analysis on the
contrast of the image (before the post-processing)
and on the Signal to Noise Ratio (SNR). The images
were recorded in the format 1024x1024pixels
2
and
the scanning frequency was set to 400 Hz. For the
optical arrangement used, the lateral and axial
resolutions for most of the measurements are R
l
=
0.375μm and R
a
= 1.4μm, while the optical slice
thickness was 2.2μm.
The deformability of the particles in the fluids
was assessed by using a 2D microfluidic device
(made of PDMS) composed of a microchannel with
a hyperbolic shaped contraction, measuring at the
end of this contraction (around the smallest cross-
section) and visualizing the particles with a high-
speed video microscopy system (high-speed camera
connected to an inverted microscope), as in Pinho
(2018).
3 RESULTS AND DISCUSSION
3.1 Design and Optimization of the
Microfluidic Device
In Vieira et al. (2017) and later in Moita et al.
(2018) a careful selection of the materials to
assemble the microfluidic device was performed,
addressing the wetting properties of the dielectric
coating. This analysis, which was performed as a
function of the dynamic response of the biofluid
droplet under electrostatic actuation revealed the
paramount role of the wetting properties of the
dielectric in allowing an efficient handling of the
biosamples. Hence, besides the hydrophobicity, the
reduced hysteresis to minimize energy dissipation at
the droplet surface contact line showed to be a factor
of major importance. Furthermore, minimizing
adsorption was also shown to be relevant, not only
to reduce contamination issues, but also to promote
droplet motion. Indeed, Moita et al. (2018) clearly
showed that the adsorption of the biocomponents
(e.g. proteins) could lead to a local increase of
the wettability, which would promote droplet
spreading and energy dissipation at the contact line,
thus limiting droplet continuous motion. In line with
this and combining the electrical properties with
the aforementioned wetting issues, Moita et al.
(2018) recommend the use of PDMS
(Polydimethylsiloxane) or Teflon coated with a
commercial compound called Glaco©, a
perfluoroalkyltrichlorosilane combined with
perfluoropolyether carboxylic acid and a fluorinated
solvent (Kato et al., 2008), to promote the
superhydrophobicity of the dielectric, while
reducing the adsorption of the biocomponents.
However, when designing the optimized
electrodes configuration, also as a function of the
dynamic response of the biosample droplets, Jacinto
et al. (2018) noticed that the negative effect of an
excessive increase of the thickness of the dielectric,
according to Young-Lippmann equation, would
reduce droplet motion, compromising the efficacy of
the microfluidic chip. It is worth mentioning that the
Young-Lippman equation states that the contact
angle of the droplet under electrostatic actuation is
proportional to the applied voltage and inversely
proportional to the thickness of the dielectric. The
detailed theoretical analysis on this and other basic
principles of electrowetting is revised in Mugele and
Baret (2005) and in Nelson and Kim (2012), for
instance.
In line with this, for the specified dielectrics, an
additional simulation was performed to optimize the
thickness of the dielectric, maximizing the resulting
electrostatic force, the distance travelled by the
droplet and its velocity. Hence, Figure 1 depicts the
numerical results on the maximum velocity of a
droplet while moving on the chip for a time interval
of 10ms, as a function of the thickness of the
dielectric, for a fixed imposed voltage of 90V.
Following the previous simulations (Jacinto et al.,
2018), the droplet is a biofluid (a solution of GFP –
Green Fluorescent Protein, produced and purified in
house, with 1.71x10
-3
mM concentration) with an
initial diameter of 1.3mm.
BIODEVICES 2019 - 12th International Conference on Biomedical Electronics and Devices
102

Figure 1: Maximum droplet velocity, as a function of the
dielectric thickness, for different dielectric materials, and a
fixed imposed voltage of 90V. The GFP droplet with
1.71x10
-3
mM concentration has an initial diameter of
1.3mm.
The Figure clearly shows the maximization of
droplet velocity, for a fixed reduced value of the
imposed voltage, for a dielectric thickness of the
order of 1-6μm. For thicker values, the droplet
velocity becomes very low, as shown in the Figure
and eventually the motion of the droplet is totally
precluded. On the other hand, the thickness can be
further tuned and eventually reduced within the
nanometric scale, although in this case, the eventual
benefits arriving for droplet transport are probably
overcome by the difficulty and costs of the
manufacturing process. Furthermore, for sub-
micrometer thickness values, dielectric breakdown is
more likely to occur, leading to the occurrence of
droplet hydrolysis, which, besides destroying the
sample droplets, can cause substantial damages in
the microfluidic chip.
Based on these final simulations and following
the previous recommendations of Vieira et al.,
(2017), Moita et al. (2018) and Jacinto et al.
(2018), the final microfluidic chips should be
coated with Teflon with a thickness between 1 and
6μm, which allows an efficient droplet motion with
velocities up to 75mm/s, for a low applied voltage
(bellow 90V) and low frequencies (9Hz). A
simplified version of the device is schematically
represented in Figure 2.
Figure 2: Simplified schematic of the microfluidic chip.
Currently only the basic chips with the optimized
electrodes size and configuration are being fabricated.
3.2 Analysis of the Suitability of the
Analogue Fluids
The analogue fluids tested here are intended to
mimic the actual biofluids to be assayed, namely in
terms of the rheological properties, deformability
and size of the particles. Despite having a shear-
thinning behaviour, close to that of blood, the
xanthan gum solutions cannot mimic the potential
rheological modifications caused by cells
deformation. In this context the polymeric
suspensions provide a much more realistic approach.
However, looking at the particles distribution within
the fluid, with the confocal microscope, the images
show a strong trend for the PMMA particles to
agglomerate forming large rigid clusters, as
illustrated in Figure 3.
Figure 3: Illustrative image of the PMMA solution (with
particles of characteristic size of 5μm) taken with the
Laser Scanning Confocal Microscope (Leica SP8). The
images were taken with an objective with 20X
magnification and with a numerical aperture of 0.75.
Cell Deformability Studies for Clinical Diagnostics: Tests with Blood Analogue Fluids using a Drop based Microfluidic Device
103

For instance in the case illustrated in Figure 3,
the characteristic size of the resulting particles is
5.46±0.38μm, but the agglomerates can be larger
than 40μm. Also, the particles are approximately
spherical, but the resulting agglomerates can depict
quite irregular shapes.
On the other hand, the analogue fluid resulting
from the addition of the surfactant is much more
interesting in terms of the particles distribution,
showing no evident agglomeration of the particles
(Figure 4a), which also depict a spherical shape. The
particle size distribution (which was evaluated for a
sample of 170 particles) is slightly heterogeneous, as
a)
b)
Figure 4: a) Representative image of the analogue fluid
prepared with the surfactant Brij40, obtained by Laser
Scanning Fluorescent Confocal Microscopy (objective of
20x magnification and 0.75x numerical aperture). b) Size
distribution of the semi-rigid particles obtained by image
post-processing.
depicted in Figure 4b), but a simple filtering of the
solution seems to considerably narrow the size
distribution, homogenising the solution (Figure 5).
It is worth mentioning that having a particle size
distribution more heterogeneous is in fact an
advantage for the current study, since the pleural
fluid samples may have different cells, with different
morphologies, being the size distribution obtained
here, similar to that reported by Tse et al. (2013)
using pleural effusions.
The complete characterization of this kind of
solutions is out of the scope of the present study and
will be presented in a future work.
Figure 5: Representative image of the analogue fluid
prepared with the surfactant, obtained by Laser Scanning
Fluorescent Confocal Microscopy (objective of 20x
magnification and 0.75x numerical aperture) after filtering
the solution with a 10μm filter.
After this brief analysis of the particles
morphology and size distribution, it is now relevant
to briefly check on their deformability, as they
should be able to present similar stages of
deformability, comparable to those of the sample
cells. The deformation index DI, as initially defined
in Pinho et al. (2013):
DI =
L
major
- L
minor
L
major
+ L
minor
(1)
where, L
major
and L
minor
refer to major and minor
axis lengths of the cell was used to assess the
deformability of the particles.
The range of maximum DI obtained with
different particles is represented in Figure 6, which
BIODEVICES 2019 - 12th International Conference on Biomedical Electronics and Devices
104
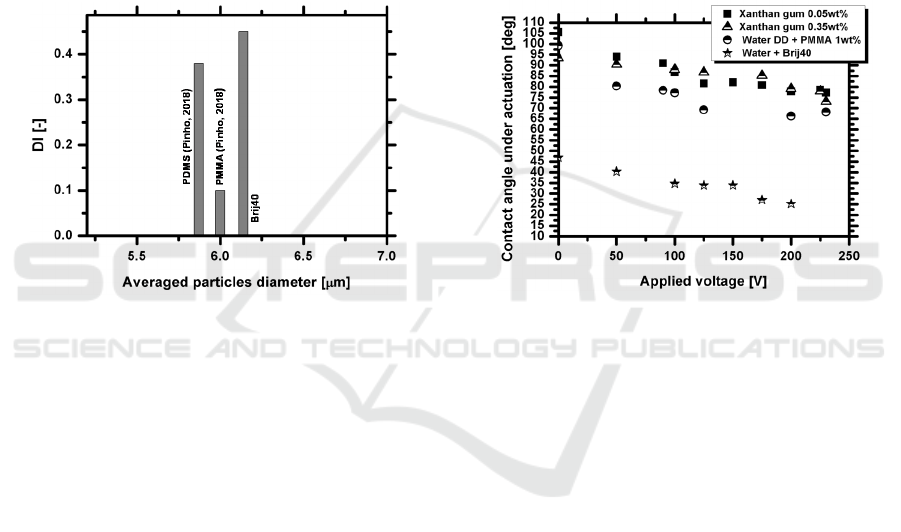
includes results gathered from the work of Pinho
(2018). The Figure shows a significant range of the
deformation index, up to 0.5, particularly for semi-
rigid particles, such as those obtained with the
surfactant solution. As reported by Pinho et al.
(2018) one may notice that the deformability of the
rigid particles such as PMMA is lower than that of
other analogues, including the semi-rigid particles
obtained with the surfactant solution. Although the
sizes of the particles used here are relatively narrow,
the analogue solution with the surfactant can provide
a wider range of particle diameters, as shown in
Figure 4b). The detailed study of the particles
deformability is out of the scope of the present work
and will be presented in a different work.
Figure 6: Deformation index DI as a function of the initial
(averaged) particles diameter for the particles used in
different analogue fluids.
The methodology used by Tse et al. (2013) for
malignant diagnostics is quite complex and requires
the establishment of different profiles, which are
mainly different distributions of the deformability
(which Tse et al., 2013 defined as the ration
L
major
/L
minor
), which is represented as a function of
the initial diameter of the cells. Overall, considering
that cell diameters in Tse et al. (2013) were ranging
between 1 and 25μm, this range of diameters is
covered with our analogues, which show
deformability ranges of the order of 1.25 or higher.
This analysis must be adapted for our case study and
for the various strategies that will be used to deform
the cells, but these preliminary results suggest a
good potential of these analogue fluids, and
particularly the Brij40 solution to be used in our
deformability studies.
Finally, given that our microfluidic device works
under electrostatic actuation it is worth to analyse
the electrostatic response of the analogue fluids. In
this context Figure 7 depicts the variation of the
equilibrium contact angle under actuation, as a
function of the applied voltage. The measurements
were performed on a Teflon substrate, as described
in section 2.2.1.
Figure 7 shows a clear response of all the
analogue fluids tested here, under electrostatic
actuation, as the contact angle decreases with the
applied voltage, according to Young-Lippmann
equation. The curves obtained here do not follow
exactly Young-Lippmann equation since this classic
theory does not take into account various
phenomena such as energy dissipation and contact
line saturation. These curves are in good qualitative
agreement with those reported by Moita et al. (2016)
taken with biofluids at similar experimental
conditions.
Figure 7: Electrostatic response of the analogue fluids:
contact angle of an actuated droplet (3μl of volume)
deposited on a Teflon substrate, as a function of the
applied voltage.
It is worth mentioning that the surfactant Brij40
solved in water significantly decreases the surface
tension of the solution (Table 1), so the contact
angles are much lower than those obtained with the
other analogue fluids, which have surface tension
values much closer to that of water. However, the
dynamic response of the Brij40 solution to the
electrostatic actuation is similar in magnitude to that
depicted by the other analogue fluids, showing no
evident signs of contact angle saturation for the
highest applied voltages, contrarily to what is
observed for instance in the xanthan gum solutions.
Following these previous results, the Brij40 solution
shows a high potential to be used as an analogue
fluid in our study.
4 CONCLUSIONS
Following our previous work, this paper addresses
the various steps required in the development of a
Cell Deformability Studies for Clinical Diagnostics: Tests with Blood Analogue Fluids using a Drop based Microfluidic Device
105

droplet based microfluidic device (based on
electrostatic actuation) for early staged cancer
diagnostics. The first part of the paper summarizes
the steps followed up to now, towards the design and
test of the microfluidic chip and discusses the final
tests on the optimization of the materials, namely of
the dielectric to be used as a coating material to our
chip. Adsorption of the biomaterials has shown to be
a relevant issue in our previous work. So, to
overcome this problem, several analogue fluids are
proposed and tested here, in an original approach, to
infer on their suitability to be used in the test of the
microfluidic device. The analogue fluids are
characterized in terms of their main physico-
chemical properties, the size distribution of the
particles (mimicking the cells) and on their
deformability, since the microfluidic device under
development will explore the potential use of cell
deformability to cancer diagnostics. The preliminary
results discussed here suggest that a surfactant
solution can be used as an analogue. The addition of
the surfactant leads to the formation of semi-rigid
particles with a size distribution (obtained by post-
processing of images taken using Laser Scanning
Fluorescent Confocal Microscopy), and
deformability characteristics compatible with those
of the biosamples to be studied.
ACKNOWLEDGEMENTS
The authors are grateful to Fundação para a Ciência
e a Tecnologia (FCT) for financing the contract of
A.S. Moita through the IF 2015 recruitment program
(IF 00810-2015) and for partially financing this
research through the exploratory project associated
to this contract.
REFERENCES
Chim, J. C. R. M., 2015. Capillary biochip for point of use
biomedical application, MSc Thesis, Instituto Superior
Técnico, Universidade de Lisboa.
Cross, M. M., 1965. Rheology of non-Newtonian fluids: a
new flow equation for pseudoplastic systems, J.
Colloid Sci., 20:417-437.
Dance, A., 2017. The making of a medical microchip.
Nature, 545:512-514.
Geng, Hongyao, Feng, J., Stabryl, L. M., Cho, S. K., 2017
Dielectroetting manipulation for digital microfluidics:
creating, transporting, splitting, and merging droplets.
Lab-on-Chip, 17:1060-1068.
Gossett, D., Weaver, W., Mach, A., Hur, S., Tse, H., Lee,
W., Amini H., Carlo, D., 2010. Label-free cell
separation and sorting in microfluidic systems,
Analytical and Bioanalytical Chemistry, 397:3249-
3267.
Hu, S., Wang, R., Tsang, C. M., Tsao, S. W., Sun, D.,
Lam, H. W., 2018. Revealing elasticity of largely
deformed cells flowing along confining
microchannels. RSC Adv., 8:1030-1038.
Jacinto, F., Moita, A.S., Moreira, A.L.N., 2018. Design,
test and fabrication of a droplet based microfluidic
device for clinical diagnostics. In: Proceedings of the
11
th
International Conference on Biomedical
Electronics and Devices – Volume 1: BIODEVICES
2018, 19 – 21 January, Funchal, Madeira, pp. 88-95.
ISBN 978-989-758-277-6.
Kato, M., Tanaka, A., Sasagawa, M., Adachi, H, 2008.
Durable automotive windshield coating and the use
thereof. US Patent, 8043421 B2.
Li, Y., Fu, Y. Q., Brodie, S. D., Alghane, M., Walton, A.
J., 2012. Integrated microfluidics system using surface
acoustic wave and electrowetting on dielectrics
technology. Biomicrofluidics, 6:012812.
Lin, C. C., Wang, J. H., Wu, H. W., Lee, G. B., 2010.
Microfluidic immunoassays. JALA - J. Assoc. Lab.
Autom., 15(3):253–274.
Moita, A. S., Herrmann, D, Moreira, A. L. N., 2015. Fluid
dynamic and heat transfer processes between solid
surfaces and non-Newtonian liquid droplets. Applied
Thermal Engineering, 88:33-46.
Moita, A. S., Laurência, C., Ramos, J.A., Prazeres, D. M.
F., Moreira, A. L. N., 2016. Dynamics of droplets of
biological fluids on smooth superhydrophobic surfaces
under electrostatic actuation, J. Bionic Eng., 13:220-
234.
Moita, A.S., Vieira, D., Mata, F., Moreira, A.L.N., 2018.
Microfluidic devices integrating alternative clinical
diagnostic techniques based on cell mechanical
properties. Biomedical Engineering Systems and
Technologies. Series: Communications in Computer
and Information Science, Chapter 4: Microfluidic
Devices Integrating Clinical Alternative Diagnostic
Techniques Based on Cell Mechanical Properties, N.
Peixoto et al. (Eds.), Springer International
Publishing AG, part of Springer Nature, 881, pp. 74-
93.
Mugele, F, Baret, J.C., 2005. Electrowetting: From basics
to applications. J. Phys. Condensed Mat., 17:R705–
R774.
Nelson, W.C., Kim, C.-j., 2012. Droplet actuation by
Electrowetting-on-Dielectric (EWOD): A review. J.
Adhesion Sci. Tech., 26, 1747-1771.
Pinho, D. M. D., 2018. Blood rheology and red blood cell
migration in microchannel flow. PhD Thesis.
Faculdade de Engenharia da Universidade do Porto.
Pinho, D., Yaginuma, T., Lima, R., 2013. A microfluidic
device for partial cell separation and deformability
assessment. BioChip Journal, 7(4):pp 367-374.
Pollack, M. G., Pamula, V.K., Srinivasan, V., Eckhardt,
A.E., 2011. Applications of electrowetting-based
digital microfluidics in clinical diagnostics, Expert
Ver. Molecular Diagnostics, 11(4):397-407.
BIODEVICES 2019 - 12th International Conference on Biomedical Electronics and Devices
106

Restolho, J., Mata, J. L., Saramago, B., 2009.
Electrowetting of ionic liquids: contact angle
saturation and irreversibility. J. Phys. Chem C, 113:
9321–9327.
Shields I. V., Reyes, C. D., López, G. P., 2015.
Microfluidic cell sorting: A review of the advances in
the separation of cells from debulking to rare cell
isolation, Lab on a Chip, 16:1230–1249.
Takahashi, K., Hattori, A., Suzuki, I., Ichiki, T., 2004.
Non-destructive on-chip cell sorting system with real-
time microscopic image processing, Journal of
Nanobiotechnology, 2:1-8.
Tse, H. T. K., Gossett, D. R., Moon, Y. S., Masaeli, M.,
Sohsman, M., Ying, Y., Mislick, K., Adam, R. P.,
Rao, J. Di Carlo, D., 2013. Quantitative diagnosis of
malignant pleural effusions by single-cell
mechanophenotyping, Science Translational
Medicine, 5(212): 212ra163.
Vieira, D. Mata, F., Moita, A.S., Moreira, A.L.N., 2017.
Microfluidic Prototype of a Lab-on-Chip Device for
Lung Cancer Diagnostics. Proceedings of the 10th
International Joint Conference on Biomedical
Engineering Systems and Technologies - Volume 1:
BIODEVICES, 63-68, 2017, Porto, Portugal, 21-13
February 2017. DOI: 10.5220/0006252700630068,
ISBN: 978-989-758-216-5.
Zeggari, R., Manceau, J. F., Aybeke, E. N., Yahiaoui, E.,
Boireau, E. L., 2014. Design and fabrication of an
acoustic micromixer for biological media activation,
Procedia Engineering, 87:935-938.
Cell Deformability Studies for Clinical Diagnostics: Tests with Blood Analogue Fluids using a Drop based Microfluidic Device
107
