
GNEUROPATHY: Validation Process at Clinical Environment
Claudia Quaresma
1,2,3
, Madalena Gomes
1
, Heitor Cardoso
3
, Nuno Ferreira
3
, Ricardo Vigário
1,2
,
Carla Quintão
1,2
and Micaela Fonseca
1,2,3,4
1
Departamento de Física, Faculdade de Ciências e Tecnologia, Universidade Nova de Lisboa,
2892-516 Monte da Caparica, Portugal
2
Laboratório de Instrumentação, Engenharia Biomédica e Física da Radiação (LIBPhys-UNL), Departamento de Física,
Faculdade de Ciências e Tecnologia, Universidade Nova de Lisboa, 2892-516 Monte da Caparica, Portugal
3
VR4NeuroPain, Rua Comandante António Feio nº26 1C1, 2800-255 Almada, Portugal
4
Universidade Europeia, Laureate International Universities, Estrada da Correia, nº53, 1500-210 Lisboa, Portugal
{heitor.cardoso, nuno.ferreira}@vr4neuropain.com
Keywords: Rehabilitation, Spinal Cord Injury, Evaluation, Device.
Abstract: Spinal cord injuries are one of the most traumatic situations with a major impact on a person's quality of
life. This type of injury have a extremely impact in the performance of daily life activities not only due to
motor alterations but also due to the appearance of neuropathic pain Throughout the rehabilitation process
the evaluation and intervention methodologies are not very systematic and are not personalized. Thus, to
bridge this gap, the VR4NeuroPain was developed a technology that associates virtual reality with a glove
"GNeuroPathy". The glove "GNeuroPathy" allows the collection of physiological parameters, namely to
identify the electrodermic activity (EDA) while the patient carries out activities in an immersive
environment. The main objective of this article is to present the validation process of the "GNeuroPathy" in
clinical context. "GNeuroPathy" was applied to a group of 17 individuals with incomplete spinal cord
injury. The results showed that "GNeuroPathy" is easy to apply and is suitable for comfort and texture. Data
were also collected from EDA and it was found that there is a significant difference in signal amplitude in
patients with low and high functionality.
1 INTRODUCTION
Spinal cord injury (SCI) is one of the most
devastating neurological injuries, as the spinal cord
is the main communication route between the brain
and the rest of the body, so injuries at this level are
devastating to the patient, both physically and
psychologically. SCI can occur following trauma to
spinal cord and also because of a variety of
pathologies (e.g. congenital, transverse myelitis,
spinal meningitis) SCI leads to dramatic losses in
neurons and synaptic connections, and consequently
function. Worldwide, the incidence of SCI ranges
from 3.6 to 195 per million (Massetti and Stein,
2018) leading to a major medical problem because
currently there is no way to repair the central
nervous system and restore function.
The long-term disability from SCI results not
only from the initial loss of function but also from
the complications that accumulate (such as severe
spasticity, infections, osteoporosis and pathologic
bone fractures) (Jazayeri et al., 2015; McDonald and
Sadowsky, 2002). A major long-term complication
is muscle wasting, where rehabilitation plays a
crucial role in the restoration and/or maintenance of
motor skills. These disabilities affect patients’ ability
to accomplish real-life activities of daily living
(ADLs), and often involve critical sub movements,
including reaching and/or grasping (Nathan et al.,
2009).
Despite the loss of functionality is considered a
major long-term complication, the neuropathic pain
can be determinant for the patient’s inability to
return to ADLs, production and entertainment.
Accordingly, it is imperative and crucial to
develop new technologies that have a significant
impact on the rehabilitation process of the SCI.
Virtual reality has become increasingly popular and
available being integrated into intervention
programs, such as for pain and stress reduction,
Quaresma, C., Gomes, M., Cardoso, H., Ferreira, N., Vigário, R., Quintão, C. and Fonseca, M.
GNEUROPATHY: Validation Process at Clinical Environment.
DOI: 10.5220/0007579702750279
In Proceedings of the 12th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2019), pages 275-279
ISBN: 978-989-758-353-7
Copyright
c
2019 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
275
skills training and rehabilitation (Chen et al., 2009).
Therefore, an innovative solution was created
called "VR4NeuroPain", which associates virtual
reality with sensory and motor stimulation
(Quaresma et al., 2018). The system consists of two
components: virtual scenarios and a glove -
"GNeuroPathy" - monitors electrophysiological data
in real time.
With the aim of providing patients with an
innovative environment for the rehabilitation
process. The "VR4NeuroPain" system allows
patients to have contact with an immersive
environment that aims to (Quaresma, et al., 2018):
● motivate for the rehabilitation process;
● play an active role in the rehabilitation process;
● promote quality of life and well-being;
● control the achievement of fine and global
movement;
● distinguish tactile sensory stimuli;
● stimulate technological literacy.
For that reason, the use of interactive technologies in
a rehabilitation process allows to reduce the time
spent in that process and greater economic
sustainability of the units of the health sector. In
order to guarantee the applicability of the system it
is necessary to carry out the validation of all the
components. Thus, "GNeuroPathy" has already been
applied in people with no associated pathology and
it has been found to be easy to apply and meets the
proposed objectives.
The present work has as main objective to
present the validation process of the glove
"GNeuroPathy" in clinical context.
2 MATERIALS AND METHODS
The study was approved by the Portuguese Ethics
Committee of the Medicine and Rehabilitation
Center of Alcoitão, in Portugal. Each participating
subject was informed about the procedures and the
objectives of the study, prior data collection, and
signed a consent form with this information.
All data was collected, during 1 month, from a
cohort of patients with spinal cord injury attending
the occupational therapy department, at the
Medicine and Rehabilitation Center of Alcoitão. The
inclusion criteria for the present study were that each
patient had incomplete spinal cord injury.
The process of validating "GNeuroPathy", in
clinical context, is divided in three parts:
1. Usability – examines the subject's degree of
satisfaction, when using the glove;
2. Data collection procedure – assesses the
performance of the protocol;
3. Data analysis – prototyping of the analysis
procedure, and the interpretation of its outcomes.
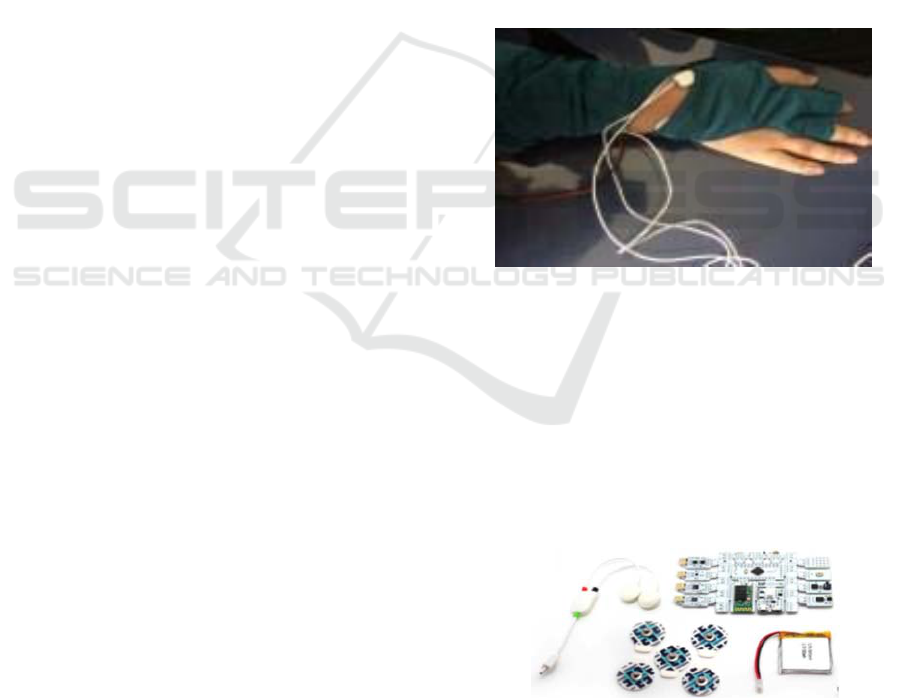
Glove “GNeuroPathy” System.
The "GNeuroPathy" glove (Figure 1) is easy to put
on, allows object manipulations and currently
integrates two types of sensors that collect electro-
dermal activity (EDA) and muscle activity (EMG)
data. To record the EMG and EDA signals, a
Bitalino acquisition module; a pair of EMG sensors
and another pair of EDA sensors were used. To
connect the sensors to the subject, 2 Ag / AgCL with
adhesive electrodes stabilized with solid adhesive
were used for each sensor (TIGA-MED Gold 01-
7500, TIGA-MED GMBH, Germany) (Guerreiro et
al., 2013; Guerreiro et al., 2014).
Figure 1: The glove "GNeuroPathy".
Bitalino, together with the sensors and the
electrodes used are shown in Figure 2. The recording
device collects the biological signals simultaneously,
with a 16-bit resolution and sampling frequencies of
up to 1000 Hz. All data is transmitted, via Bluetooth,
from Bitalino to the computer for processing. In the
latter, the software used was Plux’s proprietary
OpenSignals (Guerreiro et al., 2013; Guerreiro et al.,
2014).
Figure 2: The components of the Bitalino and the EDA
sensors (Guerreiro et al., 2013; Guerreiro et al., 2014).
Glove “GNeuroPathy” Data Collection.
After installing the glove, with all sensors securely
BIODEVICES 2019 - 12th International Conference on Biomedical Electronics and Devices
276
attached to the skin, and the hand held by the
therapist, stimulation was applied to the hand, at the
level of the fingers, in a pre-determined sequence.
The first stimulus consisted of a touch, with a pin, to
the hand. Subsequent stimuli consisted of sandpaper,
cotton, hot and cold contacts with the skin. To
reduce the psychological effects of stimulus
preparation, the subject was asked to look in the
opposite direction to the stimulated hand
The data collection procedure is shown in Figure
3.
Each stimulus was applied for 10 seconds.
During the application of each stimulus, EDA
parameters were collected. Although both EDA and
EMG could have been recorded, in connection with
the use of the “GNeuroPathy” glove, this
preliminary, validation work collected only EDA
information from all subjects. Three collections were
made for each stimulus type. The goal was to assess
the robustness of the data collected, as well as
possible habituation effects, associated with the
repeated application of each stimulus type.
Figure 3: The figure shows the data collection protocol.
Characterization of the Sample.
The sample consisted of 17 patients (7 women and
10 men), aged between 22 and 81 years, with an
average age of 55 +/-18 years and an average body
mass index of 26 +/- 4. Seven patients did not report
having pain; and the remaining ten scored, in the
visual analogue scale (Boonstra et al., 2008), as
shown in Figure 4.
Figure 4: Reported pain for the subjects participating in
the study, following the visual analogue scale (Boonstra et
al., 2008).
Validation of Data Collection Procedure.
The following data collection protocol was applied
to all patients:
1. Describe the study’s objectives and obtain the
informed consent;
2. Collect demographic data from the participants;
3. Placement of the electrodes on the anterior face
of the right hand. A grounding electrode is also
placed in the styloid process of the ulna;
4. Placing the glove;
5. Data collection, following the protocol described
in Figure 3;
6. Removal of the electrodes and glove
7. Fill patient’s satisfaction questionnaire,
regarding the use of the glove.
3 RESULTS
Usability Tests.
These tests were conducted to evaluate the
parameters of Visual aspect; Accessibility in place;
Comfort; Fixation and Texture, in a scale where
each of them was considered as Unsuitable, Partially
adequate or Suitable.
During the usability tests it was found that 94%
of all participating subjects considered
"GNeuroPathy" to be "adequate", from visual
appearance and comfort perspectives. In addition,
65% of all patients recommended the use of
GNEUROPATHY: Validation Process at Clinical Environment
277

Different type of stimuli
"GNeuroPathy", and 82% reported that its fixation is
"adequate". None of the patients considered the
device “Unsuitable”.
Figure 5: Patients' degree of satisfaction.
Clinical Observations.
After validating the usability of the GNeuroPathy
device, physiological data was collected with it. The
duration of one full study, running over all stimuli
within the protocol, lasted not more than 10 minutes.
The recorded EDA consisted of averages of the
electrodermal activity, within a fixed temporal
window length, after the application of the
respective stimulus (Figure 6).
Figure 6: Values of averaged EDA, for all stimuli types,
and three different groups of patients suffering from
incomplete spinal cord injury. For comparison, also the
values found from subjects reporting no level of
pathological pain.
We observed that, throughout all patients, the
EDA means changed rather highly. From the 17
subjects, about 5 of those presented very low values,
when compared to a similar study performed on
healthy subjects (Quaresma et al., 2018)). In
addition, 5 had close to “normal” EDA values. The
remaining 7 had values spreading from one end to
the other. Hence, we divided our subjects according
to those characteristics, as summarized in Figure 6.
Two significant considerations may be drawn
from the results reported. In an immediate look, it is
clear that any type of stimulation seems to result in
an increase in EDA response higher than the basal
response, ie., a condition where no stimulation
occurred. In addition, the “hot” stimulus produced
the highest response, whereas all others seem rather
similar to one another.
The second, and possibly the most important
result, is that the five patients with the most severe
functional limitations, represented in the graph with
a blue color, displayed the lowest EDA values. Even
the group with mild limitations, in red, have values
that are considerably lower than the ones displayed
by the healthy group. Finally, patients with rather
good functionality differed little from the group of
healthy subjects – both in EDA values themselves,
as well as in the relative magnitude variation with
the type of stimulus employed.
4 CONCLUSIONS
The principal objective of this article is to present de
validation process in a clinical context.
For this
purpose, the glove "GNeuroPathy" was utilized in a
study comprising 17 patients with incomplete spinal
cord injury, to collect EDA data. In addition, a
usability test was also performed.
This research is part of an ongoing project for
system development, called "VR4NeuroPain". In
this article, the tests performed on the glove
prototype "GNeuroPathy", the physical element of
the "VR4NeuroPain" system, were presented.
This study contributed to obtaining a clear
feedback on the design and usability of the
prototype. The data collection procedure, in the
context of EDA response to external stimulation of
the hand was also tested.
Although outside of the main purposes of this
work, we have observed that EDA is a good
indicator of the level of functionality in patients with
incomplete spinal cord injuries. As such, one may
foresee that a device such as “GNeuroPathy” may be
employed to help diagnosing said disease, as well as
assess the benefits of given rehabilitation
interventions.
In the future we will also develop a software
platform where to add all algorithms required for
physiological signal processing. In addition, the
glove – "GNeuroPathy" – must also be validated
associated with the other parts of the system. Tests
BIODEVICES 2019 - 12th International Conference on Biomedical Electronics and Devices
278

in individuals with neuropathic pain will be
performed within the "VR4NeuroPain" system, and
compared with the conventional procedures, in order
to prove the reliability of the system.
The system can be used by multiple users, such
us physicians and occupational therapists, and will
allow us to apply innovative and interactive
methodologies of intervention, promoting the
process of rehabilitation.
ACKNOWLEDGEMENTS
The authors would like to thank all the healthcare
professionals of Medicine and Rehabilitation Center of
Alcoitão The authors would like to thank Collide for the
help and support provided in this investigation.
REFERENCES
Massetti J. & Stein D.M., 2018. Spinal Cord Injury. White
J., Sheth K. (eds) Neurocritical Care for the Advanced
Practice Clinician. Springer, Cham.
Jazayeri S.B., Beygi S., Shokraneh F., Hagen E.M.,
Rahimi-Movaghar V., 2015. Incidence of traumatic
spinal cord injury worldwide: a systematic review. Eur
Spine J. 24(5):905–18.
Spinal-cord injury. W. McDonald W. J., Sadowsky
C.2002. Spinal-cord injury. Lancet. 359(9304): 417–
425.
Nathan, D., Johnson, M., McGuire, J.R., 2009. Design and
validation of low-cost assistive glove for hand
assessment and therapy during activity of daily living-
focused robotic stroke therapy. J. Rehabil. Res. Dev.
46(5), 587–602.
Chen, C., Jeng, M., Fung, C., Doong, J., Chuang, T.-Y.,
2009. Psychological benefits of virtual reality for
patients in rehabilitation therapy. J. Sport Rehabil. 18,
258–268.
Quaresma, C., Gomes, M., Cardoso, H., Ferreira N.,
Vigário, R., Quintão C., and Fonseca, M., (2018). An
Integrated System Combining Virtual Reality with a
Glove with Biosensors for Neuropathic Pain: A
Concept Validation. AHFE 2018, AISC 781, pp. 274–
284.
Boonstra, A., Schiphorst Preuper, H., Reneman, M.,
Posthumus, J., Stewart, R., 2008 Reliability and
validity of the visual analogue scale for disability in
patients with chronic musculoskeletal pain.
International Journal of Rehabilitation Research: 31
(2): 165-169.
Guerreiro, J., Martins, R., Silva, H., Lourenço, A., Fred,
A., 2013 BITalino: A Multimodal Platform for
Physiological Computing. Proc of the Int’l Conference
on Informatics in Control, Automation and Robotics
(ICINCO): 500–506.
Guerreiro, J., Lourenço A., Silva H., Fred A. 2014.
Performance Comparison of Low-cost Hardware
Platforms Targeting Physiological Computing
Applications. Conference on Electronics,
Telecommunications and Computers – CETC 2013
Procedia Technology 17: 399 – 406.
GNEUROPATHY: Validation Process at Clinical Environment
279
