
Intrauterine HIFU: A New Treatment for Uterine Fibroids
Marie-Caroline Faisant
1
, Benzion Amoyav
2
, Quentin Perrier
3
, Laura Eling
4
and Jayashree Rangaraja
5
1
Grenoble-Alpes University Hospital, University Gynaecology and Obstetrics Department, F-38000 Grenoble, France
2
The Institute for Drug Research, The School of Pharmacy, The Hebrew University of Jerusalem, Jerusalem, Israel
3
Grenoble-Alpes University Hospital, University Pharmacy Department, F-38000 Grenoble, France
4
Rayonnement Synchrotron et Recherche Médicale, Université Grenoble Alpes, F-38000 Grenoble, France
5
Friedrich-Alexander University of Erlangen-Nuernberg, Erlangen, Germany
Keywords: Uterine Fibroid, HIFU (High Intensity Focused Ultrasound).
Abstract: Uterine fibroids are the most common benign tumours among women of child- bearing age with a prevalence
varying from 5.4% to 77%. The most common symptoms of uterine fibroids include: heavy menstrual
bleeding, pelvic pressure or pain and infertility. Symptoms can be influenced by the location, size and number
of fibroids. Fibroids are most often identified during a routine pelvic examination by a physician. Treatments
may include temporary medical treatments, surgery or uterine artery embolization. As all of the methods
above have considerable disadvantages, we propose a medical device using High Intensity Focused
Ultrasound (HIFU) in a minimally procedure in which an ultrasound probe is inserted into the uterus to
precisely ablate the fibroid and shrink it. Our new medical device proposal is aimed at improving clinical
efficacy and patient satisfaction.
1 INTRODUCTION
We are a team of biomedical engineers, pharmacists
and physicians including a gynaecologist who have
met during the Clinmed 2018 Summer School
organised by the EIT Health committee and the CIC-
IT network (Clinical Investigation Centre -
Technologic Innovation).
The Clinmed 2018 Summer School was an
immersive experience with the opportunity to meet
other students from various fields, assist to lectures
and workshops. It aimed at understanding the unmet
needs in healthcare industry and finally proposing a
feasible technological innovation from initial concept
till final development. Several teams were created
and associated to a specific CIC-IT centre. Our team
was located in Grenoble and supervised by Pr
Alexandre Moreau-Gaudry. Our goal was to create an
innovative medical device, in a two-week period,
during the summer school.
We address a current problem in gynaecology,
called uterine fibroids, which is widely known and
concerns a majority of women around the world. The
idea arose after interactions with healthcare
professionals and surgeons at the University Hospital
of Grenoble. The presentation of modern robots for
knee surgery kindled our interest on computer
assisted medical procedures and out of our expertise
we discovered the lack of efficient and less invasive
treatment of uterine fibroids in women. To overcome
important limits concerning sterilization and
maintenance of medical devices, the sterilization and
biomedical engineering department at the hospital
increased our awareness of today’s needs in health
care industry. Since health care-associated infections
are a non-negligible risk, we propose an innovation
that will reduce surgical site infection and take full
advantage of advanced computer technology to guide
the physician to target the fibroid and to ablate it.
Uterine fibroid is the most common benign
tumour among women. They occur in almost 70 % of
Caucasian women and in more than 80 % of African
American women by age 40 and a third of them are
symptomatic (Baird et al, 2003) (Khan and al, 2014)
(Stewart et al, 2017). Symptoms are heavy and long
menstrual bleeding, acute and chronic pelvic pain,
infertility, anaemia, urinary and digestive symptoms
(constipation). Symptoms depend on the localization
of the uterine fibroid as classified by the international
FIGO system.
Faisant, M., Amoyav, B., Perrier, Q., Eling, L. and Rangaraja, J.
Intrauterine HIFU: A New Treatment for Uterine Fibroids.
DOI: 10.5220/0007695205950602
In Proceedings of the 12th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2019), pages 595-602
ISBN: 978-989-758-353-7
Copyright
c
2019 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
595

2 TREATMENT OPTIONS
2.1 Current Treatment Options
2.1.1 Medical Treatment
Medical treatments for fibroids are only symptomatic
or temporary treatments. NSAIDS (non-steroidal
anti-inflammatory drugs) are used to treat acute
pelvic pain and tranexamic acid to treat heavy
bleedings. Hormonal treatments (GnRH) can be used
on a 3 to 6 months period or to prepare surgery.
Acetate ulispristal which induces apoptosis in
fibroid cells and therefore reduce significantly the
fibroid size. This treatment is indicated before
surgery or for women in whom surgery is not suitable.
However, this treatment has been reviewed recently
because of reported cases of liver injury and hepatic
failure.
2.1.2 Surgical Treatment
Nowadays we can consider three main types of
surgeries: hysteroscopy, myomectomy and
hysterectomy (Chanelles et al, 2010).
Hysteroscopy is a quick and safe procedure,
however it is only applicable for fibroids which are
reachable from the cavity.
Myomectomy; the local removal of the fibroid is
not always possible since it depends on the
localization of the fibroid. It can weaken the uterus
muscle and may lead to a preventive caesarean
section in case of a future pregnancy.
A final option is the hysterectomy which is the
total removal of the uterus. Today, uterine fibroid is
the main indication for hysterectomy.
This surgery may be complicated by urinary and
digestive per-operative wounds. Furthermore, many
women refuse hysterectomy out of sensible personal
reasons.
2.1.3 Non Invasive Treatment
Other treatments which are less invasive than surgery
exist to treat fibroid. The uterine artery
embolization is performed by radiologists, however
the inflammation and necrosis induced by the
embolization lead to painful recovery. Furthermore, a
pregnancy is not possible after a uterine artery
embolization.
Radiofrequency ablation (RFA) is another option
to treat fibroids, it is quite new and not well evaluated
yet.
2.2 HIFU Technology
2.2.1 Definition
High Intensity Focused Ultrasound, known as HIFU,
is an innovative technology which could promote an
alternative in fibroid treatment (Donnez J, 2016)
(Marret et al, 2010).
Ultrasounds are vibrations delivered by an
external transducer with a very high frequency. The
produced waves deliver energy as they travel through
tissues.
However, by increasing the intensity of the waves
and focusing them, HIFU technology deposits large
amounts of energy into tissues, which leads to the
ablation of cells in a focal point
Two main mechanisms are involved: the thermal
effect and the acoustic cavitation. With regard to the
thermal effect, the absorption of ultrasounds energy
into the tissues in a few seconds (with each pulse)
causes a sharp increase in temperature higher than
70°C, which leads to cell damage. The cavitation
effect is induced by the interaction of HIFU and
micro-bubbles in the sonicated tissue, it enhances cell
membrane permeability and leads to tissue ablation.
Both of these mechanisms lead to the destruction of
the cells by a coagulative necrosis.
2.2.2 Medical Application
Since 2000, HIFU technology is well known in
various medical fields. Dermatologists use HIFU
technology to treat wrinkles, it is nowadays
considered as a safe and effective procedure to
improve skin elasticity (Ko et al, 2017). HIFU
technology is also used to treat localized prostate
cancer where a probe is introduced into the rectum
next to the prostate, so that the urologic surgeon can
target and ablate the cancer (Gelet and al, 2009).
HIFU technology has been tested on fibroids; it has
demonstrated high efficacy, however, there are
considerable limitations as shown below. The
procedure is as follows: the patient is laying on the
abdomen on a surgical table. The HIFU transducer is
situated underneath the table. The procedure is
supervised by an MRI machine which controls the
heat produced by the HIFU waves (Marret et al,
2010).
External HIFU technology efficacy is controlled
by two main factors: the acoustic intensities and the
focalization of the ultrasound waves. Power
converters are used to increase and obtain efficient
acoustic intensities (the main goal is to obtain a power
of 100W/cm²). Focalization of the ultrasounds is
possible thanks to the use of several transducers at the
ClinMed 2019 - Special Session on Designing Future Health Innovations as Needed
596
same time. The total energy released is around 7500
joules which is equivalent to a heat of 70°.
One of the main advantages of HIFU is the
theoretic preservation of fertility.
However, the HIFU technique used to date is
limited by its external use, due to the following
reasons: risk of bowel perforation of the digestive
tract, risk of skin lesion, costly associated MRI and
no reimbursement, contraindications such as
abdominal scars, localization of the fibroid (next to
the spine, intracavitary, sub-mucosal, retro-versed in
the posterior wall of the uterus) and obesity (Marret
et al, 2010) (Bohlmann et al, 2014) .
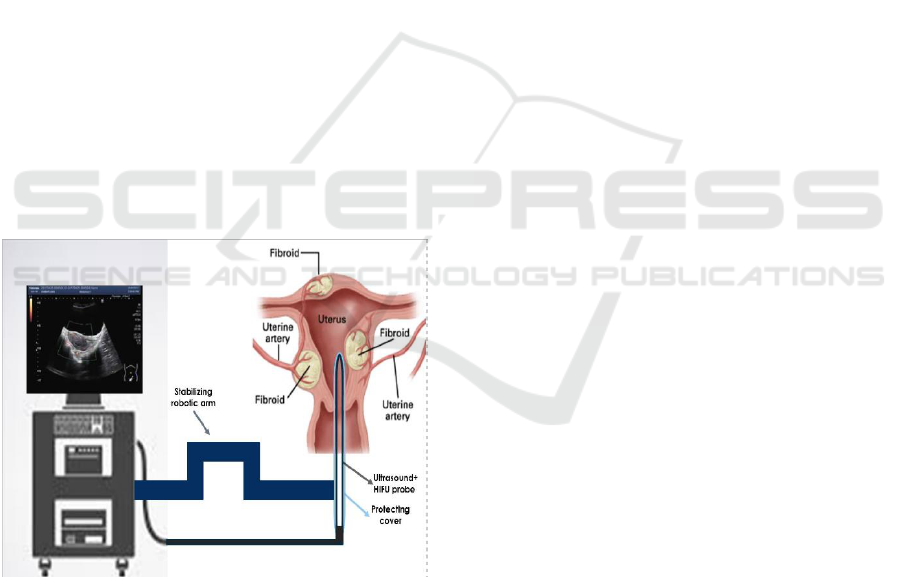
3 THE MEDICAL DEVICE
We propose an innovative medical device inserted
directly into the uterus through the vagina. The probe
combines imaging ultra sounds to target the fibroid
with HIFU to ablate and shrink it.
As illustrated, the medical device is divided into
three parts: the two functions probe, the stabilizing
arm, and the machine. The probe is the only part of
the device that needs to be sterile. Once the probe is
inserted into the uterine cavity, the surgeon can fix the
probe and leave the operative field. Then, he can
manage the fibroid ablation from the computer.
Figure 1: Schematic illustration of the device.
3.1 The Probe
The probe will be made of stainless steel and will
include the imaging ultrasound device and the HIFU
transducer.
3.1.1 The Imaging Ultrasound
It consists of an ultrasound device which allows
precise real-time mapping and 3D reconstruction of
the organ including the fibroid. The treatment and
imaging process are interleaved to allow visual
tracking of the ablation.
This can be achieved by elastography and the
change of the speed of ultrasound waves due to
temperature change (Rueff & Raman, 2013)
(Tavakkoli & Sanghvi, 2011).
3.1.2 The Transducer
In the same probe a transducer is included which
allows HIFU ablation of the targeted tissue. The
ablation of the targeted tissue can be achieved due to
the thermic and the mechanic effect of HIFU (Donnez
J, 2016) (Rueff & Raman, 2013)
leading to a
coagulative tissue necrosis. The transducer is located
laterally and is approximately 6 cm long and 1 cm
wide. As explained above, we need several transducer
units to focalize the ultrasounds.
We will target the same goals as the external
HIFU technology: obtaining a power of 100W/cm²,
a total energy release of around 7500 joules which is
equivalent to a heat of 70°. This is a challenging
objective as we need to miniaturise the technology
currently used for HIFU technology. A first step
would be to try to adjust the frequency, the range and
the throb of the ultrasound waves. A second step
would be to work on various probe components to
find the one which maximises HIFU technology.
The transducer will be made of hard ceramic
piezoelectric material (e.g. titanium, nickel), that
characterized with low dielectric losses and
biocompatibility. This means that the probe can be
driven to higher frequencies and voltages, without
causing self-heating of the transducer (Vijaya, 2013).
For the time being, our device is limited to
laterally located fibroids, but we plan to create a
device which will reach any localization in the future.
Alternatively, we consider two different types of
HIFU transducer, one located laterally and one
located on top of the probe.
As illustrated below, we have planned to create a
probe with ceramic, within the probe, we need to
insert several transducers, the amplifier and the driver
pulse. The challenge is to obtain a 1 cm wide probe to
respect the uterine cervix.
Intrauterine HIFU: A New Treatment for Uterine Fibroids
597
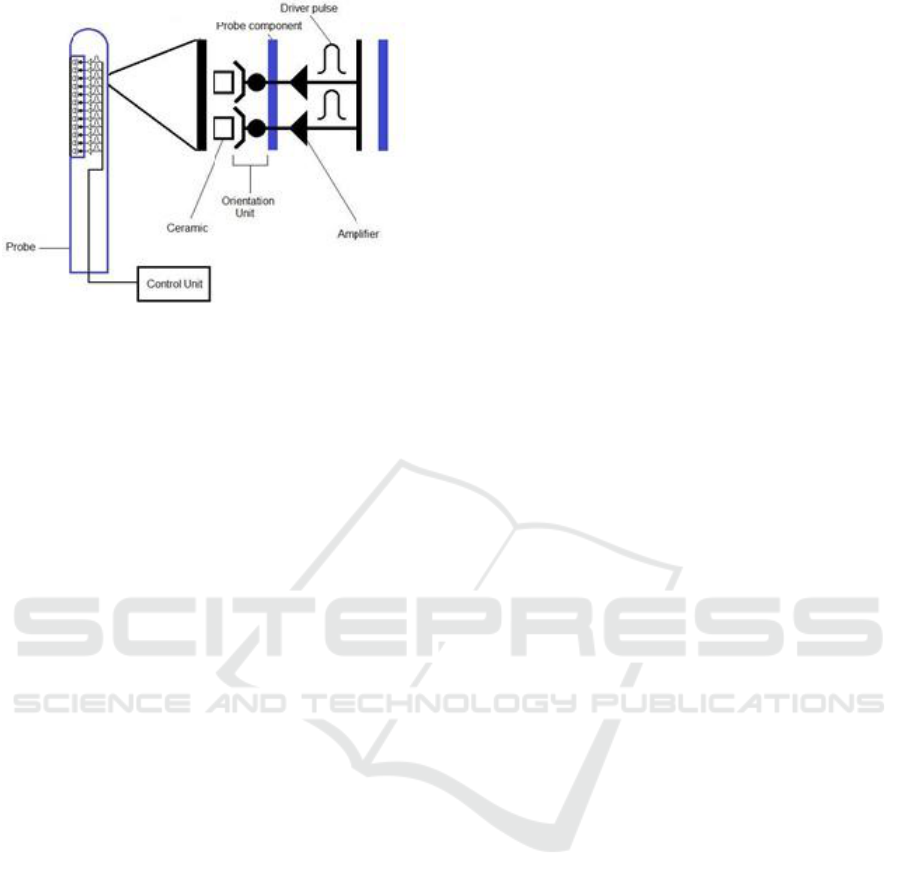
Figure 2: Schematic illustration of the probe.
3.1.3 The Protective Cover
Due to the fact that the probe is inserted into a sterile
environment, it has to be covered with a sterile
disposable cover made of transparent and
biocompatible material which allows the transduction
of ultrasounds.
To allow the transduction of the ultrasounds, the
cover will be filled with and covered in conductive
gel.
To define the exact composition of the cover
material, the first lead is the plastic which is already
used for intra uterine cannulas (used in case of
aspiration curettage). Other sterile, latex-free covers
which are on the market are made of polyethylene but
are limited in our case due to the fragility and
therefore high risk of infection.
3.2 The Machine
3.2.1 The Screen
The screen allows the visualization of a 3D image of
the target and a colour code during the treatment in
accordance to the efficacy of the ablation (turning red
to green once the tissue is successfully ablated).
3.2.2 The Software
Contouring of the fibroid is done by the surgeon and
its precise localization in the uterus can be achieved
by the software. The energy and power level of the
HIFU pulses will be managed by the software.
3.2.3 The Arm
Since the treatment time may be long depending on
the fibroid size, the probe will be connected to a
stabilizing arm once it is set in place by the surgeon.
3.3 User Guide
A user guide will be provided with the device.
We recommend a gynaecologic positioning for
the patient for a better use of the probe.
A disinfection of the vagina and the use of an
operative field are necessary to reduce the risk of
infection.
A dilatation of the cervix to 11 mm is necessary
to insert the probe with its sterile cover.
Once the probe is in place, the surgeon uses the probe
to target the fibroid. The probe is then stabilized by
the arm and the HIFU ablation may start.
The patient should be fasting and we recommend
the placement of a urinary catheter to protect the
bladder.
Since the procedure is minimally invasive, the
patient is free to leave the same day without
hospitalization. It is expected that the recovery phase
will not take more than a few days, which is why sick
leave at work is kept at a minimum.
3.4 Advantages and Limitations
3.4.1 Advantages
With our device it is possible to avoid MRI control
during the HIFU ablation which therefore reduces the
cost of the operation.
It will be possible to treat fibroid in any
localization in the uterus since the probe is inserted in
close contact to the target. We will be able to treat
fibroids up to the size of 10 cm.
Contrary to HIFU external use, the risks of skin
irritation and bowel perforation can be considerably
decreased. Furthermore, the treatment of obese
women and women who already underwent
abdominal surgery, leading to scar tissue, is not a
restriction.
Compared to external HIFU we are closer to our
target and can therefore increase ultrasound
frequency. This way the sound waves are absorbed
faster (low penetration depth due to absorption)
which could increase the speed of treatment.
The patient will undergo general or epidural
anaesthesia during the procedure.
As the uterus is in the pelvis, the impact of the
patient breathing movements should be non-
significant. The bladder is kept empty thanks to thE
urinary catheter. Finally, the stabilizing arm
maintains the probe in the good position.
ClinMed 2019 - Special Session on Designing Future Health Innovations as Needed
598
3.4.2 Limitations
There are still limitations which are common with all
the current surgical treatments such as infections
(endometriosis, vaginitis, urinary active infection)
and malignant cervix tumour.
Furthermore, patients with uterine malformation,
fibroids with a size over 10 cm and fibroids localized
too close to the digestive tract should not be treated
with our device.
Hyper sensibility to components of our device is
a contraindication.
4 DEVELOPMENT PLAN
4.1 Risk Analysis
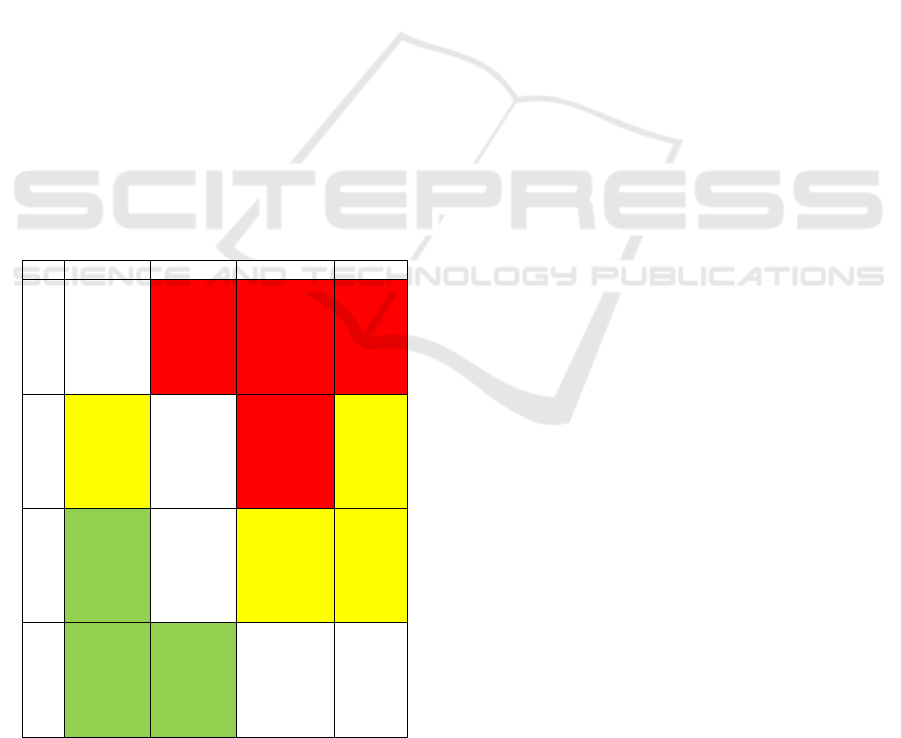
Our device is classed as a IIb medical device.
Risk assessment is very important as it is
considered as an integral part of an occupational
health and safety management plan. We have created
a table which gives an overview of the risks for our
medical device according to the new guidelines (ISO
14971). We used a severity colour code (red is
extreme severity, yellow moderate severity and green
is low severity):
Table 1: Risk analysis.
Frequent
Likely
Occasional
Rare
Catastrophic
infertility
miscar-
riage
Electric
hazard,
Uterine
synechia
perforation
Death,
anaphyl
-actic
shock
Haemor
-rhage
Critical
Damage
to organs
Software
issues
surgical
site
infection
Failure
Significa
nt
Pelvic
pain,
Hypersensi
-tivity to
component,
loss of
blood
Breach
of
sterile
cover,
scar
tissue
Minor
Nausea
and
vomiting
Fever
after
surgery
4.2 Essential Requirements
4.2.1 Biocompatibility
The probe must pass all the biocompatibility tests and
allow the focused ultrasound waves to pass through
the cover.
We will use a special designed piezoelectric
ceramic material that will address our requirements
regarding safety and efficacy.
4.2.2 Risk of Infection
To keep the infection level as low as possible; we
propose a one-time use sterile cover filled with sterile
conductive gel. After use, the probe and the robotic
arm must be disinfected.
4.2.3 Risk of Electrical Hazards
To reduce the risk of electrical hazards, we will use
insulated electric wires and backup power supply to
ensure continuity and stress free surgical procedures.
4.2.4 Safety and Usability
Every device will come with a safety and usability
manual to ensure that the involved person is well
trained to conduct the procedure (workshops to train
the physicians in technical and software knowledge
are envisioned).
4.2.5 Software Requirements
We will guaranty the maintenance of the software
regularly. It will be our responsibility to upgrade the
software with the latest cybersecurity precautions to
ensure information protection.
4.3 Usability Evaluation
4.3.1 Effectiveness
Our device offers an effective way of treating fibroids
in the uterus, it is less invasive than surgical
treatments and therefore there is less risk of infection.
4.3.2 Satisfaction
We will propose a detailed usability manual to help
physicians integrate easily with the new product.
As gynaecologist surgeons know how to use an
endovaginal (or transvaginal) ultrasound probe, we
assume the learning curve will be fast. However, it is
essential to train gynaecologist surgeons about HIFU
Intrauterine HIFU: A New Treatment for Uterine Fibroids
599

technology, this may be possible through workshop
sessions.
The procedure will be performed by the
gynaecologist surgeon, this is an innovative added
value: the surgeon can schedule directly the
procedure on the surgery program, there is no more
need for a radiologist ( as for external HIFU
technology.) As there is only one physician involved;
the procedure organization is faster, the risk of
medical error decreases and the patient trust
increases.
Intrauterine HIFU use may lead to cost savings in
terms of hospitalization (ambulatory hospitalization)
and sick leave period (we recommend a one week sick
leave whereas a three weeks sick leave is often
needed after hysterectomy, myomectomy or uterine
artery embolization).
Furthermore, it offers a psychological satisfaction
to the patient (not losing the uterus and chances of
retaining fertility, no scar). HIFU technology is more
efficient than medical treatment and less invasive
than surgical treatment. The procedure is under
general or epidural anaesthesia, this allows the
procedure to be safe and painless.
4.3.3 Efficiency
Using an internal delivery of the HIFU technology
may require less time than the external HIFU use.
Moreover, with our device, there is no need for a
radiologist; the user of the device is the gynaecologic
surgeon.
Finally, the ambulatory hospitalization makes the
procedure easier and quicker.
4.4 Clinical Trial
We will conduct the necessary pre-clinical and
clinical tests (e.g. In vitro proof-of-concept test for
HIFU transducer) by accredited laboratories,
involving the required notified body with regard to
our medical device class.
4.4.1 Phase 1: In Vitro
We plan to study HIFU treatment efficacy on fibroma
cell culture. As for the safety test, we will study the
toxicity on myometrial and endometrial cell culture.
As we want our technique to be used for women
with infertility due to fibroid, we will be extremely
focused on the impacts of HIFU on the endometrial
tissue.
4.4.2 Phase 2a: In Vivo
We will test our device on animal models (healthy
sheep whose uterus have many similarities with
human uterus). We plan to obtain and study histologic
sampling post HIFU treatment to study the long term
effects on tissues ( study of the tissue after 3 days, 14
days, 2 months, 6 months, and finally after 1 year).
For each animal 4 treatment sites will be tested
(lateral, fundus, sub-mucosal, sub-serosal).
Other animal models are limited in our case, such
as horses (commonly concerned by fibroma, but too
expensive), ruminants(less expensive, but very rare
fibroma cases), rats (not comparable to humans due
to their size)
4.4.3 Phase 2b: Human Trials – Safety and
Efficacy
Our primary outcome will be the reduction of
symptoms according to the UFS-QOL scale and
patient satisfaction (opinion survey).
Our secondary outcome will be the reduction in
menstrual blood loss (using the PBAC score), the
decrease of the fibroid size (MRI 6 month after the
procedure).
Concerning the study population; the included
subjects will be women over 42 years old or women
older than 30 years with tubal ligation. Further
patients include those suffering from significant
fibroid symptoms for more than 3 months, uterine
fibroid FIGO classification type 1 to 7 or uterine
fibroid over 10 cm of size.
Subjects to be excluded are subjects with an
urgent need for surgery, pregnant women, less than
18 years old women, patient with a desire for
pregnancy, patients with severe endometriosis, pelvic
or uncontrolled systemic disease, history of lower
abdominal surgery and MRI contraindication.
So far, the exact number of patients is not known
but we estimate a total of 60 patients.
As for the trial follow-up we plan to evaluate the
success of the treatment with a MRI at 6 months to
measure the remaining fibroid tissue. We will control
the endometrial tissue by performing a hysteroscopy.
4.4.4 Phase 3: Human Trials – Comparison
We will study subjects with fibroma suitable for
external and internal HIFU (Fibroma type 4 to 6 with
an anterior localization), who are older than 18 years
old.
We will lead a prospective randomized study;
however, it won’t be possible to double-blind the
study.
ClinMed 2019 - Special Session on Designing Future Health Innovations as Needed
600

5 CONCLUSION
Intrauterine HIFU technology is the answer to a real
need for women.
Our device is promising, not only on a technical
point of view but also regarding the business and
marketing approach. Once this step is achieved, we
plan to improve our medical device by creating an
adjustable wide-angle probe and an automated
guidance arm to increase precision of HIFU treatment
of fibroids in every localization.
More indications will allow to expand our
technology, for instance endometrial hyperplasia or
patients with malign endometrium tumour who are
contraindicated to surgery.
ACKNOWLEDGEMENTS
This work received funding from EIT-Health campus
call (Project Grant Agreement n°18497).
We would like to thank the EIT Health committee and
its partners for organising the Clinmed 2018 Summer
School where we had the chance to meet and develop
our project. This was a rewarding experience.
This would not have been possible without the CIC-
IT from Grenoble and we would like to thank Pr
Alexandre Moreau-Gaudry, Isabelle Marque, Laura
Cotarla and the whole team.
We devote a special thanks to the University Hospital
of Grenoble for welcoming us in the different
departments we have visited. We warmly thank Dr
François Istasse from the gynaecology department
who answered our various questions about uterine
fibroids.
We would also like to thank Pr Frederic Patat and Pr
Jacques Felblinger who answered to our technical
questions about HIFU technology and helped to
feature our project.
Finally, we would like to thank Adria Maceira who
besides giving us marketing advices, supported us
and added to our project a special team spirit!
REFERENCES
Baird et al, D., 2003. High cumulative incidence of uterine
leiomyoma in black and white women : ultrasound
evidence.. Am J Obstet Gynecol.
Bohlmann et al, M., 2014. High intensity focused
ultrasound ablation of uterine fibroids - potential impact
on fertility and pregnancy outcome. Geburtshilfe
Frauenheilkd. .
Chanelles et al, O., 2010. Fibromes - la chirurgie.
Recommandations CNGOF.
Donnez J, D. M., 2016. Uterine fibroid management : from
the present to the future. Hum Reprod Update.
Gelet and al, A., 2009. Transrectal High-Intensity Focused
Ultrasound: Minimally Invasive Therapy of Localized
Prostate Cancer.. Journal of Endourology.
Khan and al, T., 2014. Uterine fibroids - current
perspectives. Int J. Women Health.
Ko et al, E., 2017. Safety of non-invasive body tightening
with high-intensity focused ultrasound (hifu).. Skin res
technol..
Marret et al, H., 2010. Thermodestruction des fibromes
utérins par ultrasons focalisés : une alternative?.
Recommandations CNGOF.
Rueff, L. and Raman, S., 2013. Clinical and technical
aspects of mr-guided high intensity focused ultrasound
for treatment of symptomatic uterine fibroids. Semin
Intervent Radiol..
Stewart et al, E., 2017. Epidemiology of uterine fibroids : a
systematic review.. Obstetric. Gyneacol..
Tavakkoli, J. and Sanghvi, N., 2011. Therapeutic
Ultrasound: Mechanisms to Applications.. s.l.:s.n.
Vijaya, M., 2013. Piezoelectric Materials and Devices:
Applications in Engineering and Medical Sciences.
s.l.:CRC press.
Yoshizawa et al, S., 2017. Enhancement of high-intensity
focused ultrasound heating by short-pulse generated
cavitation.. Appl Sci.
Yu An et al, C., 2017. An ultrasound imaging-guided
robotic hifu ablation experimental system and accuracy
evaluations, applied bionics and biomechanics.. Appl
Bionics Biomech..
Intrauterine HIFU: A New Treatment for Uterine Fibroids
601
APPENDIX
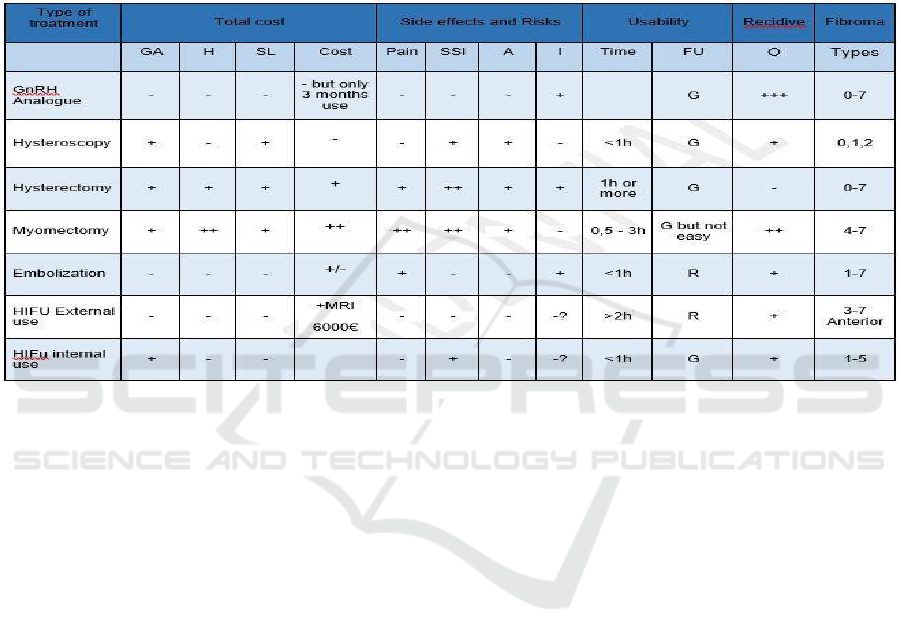
Competitors analysis :
GA = General anaesthesia
H = Hospitalization
SL = Sick leave
SSI = Surgical site infection
A = Anaemia
I = Infertility
FU = Final user, can be Gynecologist (G) or Radiologist (R) O = Occurrence
ClinMed 2019 - Special Session on Designing Future Health Innovations as Needed
602
