
Inhibitory Activity of Allium chinense G. Don. Extracts to Prodigiosin
Synthesis by Serratia Marcescens
Febry Rahmadhani Hasibuan
1
, It Jamilah
1
and Sovia Lenny
2
1
Department of Biology, Faculty of Mathematics and Natural Sciences, Universitas Sumatera Utara, Medan, Indonesia
2
Department of Chemistry, Faculty of Mathematics and Natural Sciences, Universitas Sumatera Utara, Medan, Indonesia
Keywords: Allium chinense G. Don., Prodigiosin, Serratia marcescens
Abstract: Bawang Batak (Allium chinense G. Don.) is one of native medicinal plants utilized as spices in North
Sumatera, Indonesia. The plant is known to exhibit antimicrobial activities against several bacterial
pathogens. Prospect of finding a new mechanism in combating bacterial infection through quorum-sensing
inhibition is an alternative way yet promising strategy to the overuse of antibiotics. The aim of this study is
to obtain the optimum concentration of methanolic (MeOH) and ethyl acetate (EtOAc) extracts of A.
chinense as quorum-sensing inhibitors to prodigiosin synthesis by S. marcescens. The inhibition of
prodigiosin synthesis is observed visually and measured in absorbance value (A
534
) compared to control.
The results showed that both extracts did not inhibit the growth of tested strain based on optical density
(OD
600
) among tested concentrations. The higher the concentration of MeOH and EtOAc extracts, the less
synthesis of prodigiosin by S. marcescens in the concentration of 0.3% (w/v) at the end of incubation period
(30h). The results showed that MeOH and EtOAc extract may be studied thoroughly for its possibility as
quorum-sensing inhibitor following further parameters in the future.
1 INTRODUCTION
Bawang Batak (Allium chinense G. Don.) is one of
native plant commonly cultivated by the Bataknese
in North Sumatera. Members of Allium, have also
been known as plant material in ethnobotanical
medicine. Allium chinense is distinct from kucai
(Allium tuberosum), both are commonly utilized as
food spices and medicines. Allium phytochemical
compounds have been reported to possess
antimicrobial activity to bacteria, fungi, viruses and
parasites (Kyung, 2012). Numerous antimicrobial
compounds have been identified, furan (Zanatta et
al., 2007), furfural (Sutar et al., 2012; Chai et al.,
2013), and allyl-acetone, allicin, diallyl-disulphide,
ajoene, and 3 (Allyl-trisulfanyl)-2-amino propanoic
acid (Bah et al., 2012). Allium chinense contained
majority of phytochemical groups of saponins,
flavonoids, terpenoids and steroids (Aulia, 2008).
The antimicrobial activity of bulb extract were
potential against Escherica coli, Salmonella typhi,
Staphylococcus aureus, Bacillus subtilis and
Candida albicans (Naibaho et al., 2015). In addition,
bioprospective study of the extracts as antibacterial
and antifungal activities have been intensively
studied for its application as food preservatives and
therapeutic agents against majority infection caused
by pathogenic microbes (Benkeblia dan Lanzotti,
2007).
Serratia marcescens is an opportunistic bacterial
pathogen with adaptive ability to withstand biocidal
properties from chemotherapy, immunotherapy
through resistance mechanism. Prodigiosin is a red-
pigmented compound synthesized by the species,
known as secondary metabolites from tri-pyrrole
family with prospect use as multifunctional
antibiotics, both as antibacteria and antifungi.
Pathogenicity of S.marcescens include pneumoniae,
urinary tract infection and bacteremia in
compromised host (Setiawan et al., 2017). The 16s
rRNA region of S. marcescens have been sequenced
and revealed that quorum sensing regulates the
overall pathogenicity of bacteria along with ability
to form biofilm and swarming mobility due to
serrawetin surfactant (Givskov et al., 1996 ; Givskov
et al., 1999). In addition, the species also resistant
endogenously against antibiotics like colistin and
cephalothin (Matshumura et al., 1998). The use of
antibiotic or plant antimicrobials to prevent food
spoilage and particular diseases have been practiced
82
Hasibuan, F., Jamilah, I. and Lenny, S.
Inhibitory Activity of Allium chinense G. Don. Extracts to Prodigiosin Synthesis by Serratia Marcescens.
DOI: 10.5220/0008506200820086
In Proceedings of the International Conference on Natural Resources and Technology (ICONART 2019), pages 82-86
ISBN: 978-989-758-404-6
Copyright
c
2019 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

and leading to end of antibiotic use due to antibiotic
resistances (Darshanee et al., 2011). Molecular
approach that currently gaining popularity is quorum
sensing-based inhibition or so called Quorum
Sensing Inhibitor (QSI) which directly inhibit the
virulence factor of a pathogen (Bai dan Rai, 2011).
The genetic expression during quorum sensing may
be hindered with further consequence of a non-
antibiotic resistance occurred (Dong et al., 2007;
Defoirdt et al., 2004). The underlying mechanism of
QSI is based on interruption of chemical
communication among intraspecific bacteria to
conduct quorum sensing hence disabling their
phenotypes as whole multi-species embodiment of
biofilm yet helping immune or antimicrobial
compounds to react more effective towards pathogen
(Hentzher dan Givskov, 2003, Nagy, 2010). In this
study, we reported an evaluation of bulb extract of
A.chinense as prospecive QSI phytochemicals based
on its performance towards prodigiosin synthesis by
Serratia marcescens.
2 RESEARCH METHODOLOGY
2.1 Inoculum preparation
Isolate of S.marcescens is firstly sub-cultured for
24h in Luria Bertani agar prior to laboratory test.
Isolate was collection of Department of
Microbiology, University of Sumatera Utara.
2.2 Phytochemical extraction
Bulbs of Bawang Batak (Allium chinense) were
obtained from vegetable garden in Sidikalang, North
Sumatera, Indonesia. Bulbs were separated from
foliars and roots then sliced to ± 5 mm thickness,
and then dried under aeration for 5 d until constant
weight. The dried bulbs were then mashed using a
blender and filtered to powder (Naibaho et al.,2015).
A 700 g simplisia powder was immersed into
Methanol/ MeOH 75% (v/v) as polar fraction and
Ethyl Acetate/ EtOAc 50% (v/v) as semi polar
fraction of distilled water. Each samples were
macerated for 3 d using a rotaryshaker. Macerates
were filtered and concentrated using a rotary
evaporator (Büchi® Rotavapor R-200, Sigma-
Aldrich). Both concentrated fractions were diluted
using Dimethyl sulfoxide (DMSO) for various
concentration stocks (Bai and Vittal, 2014).
2.3 Determination of QSI activity
Serratia marcescens is known to produce red
pigments namely prodigiosin in growth medium as
an indication of quorum sensing occurrence. The
measurement of prodigiosin concentration using a
spectrophotometer at a wavelength of 534 nm (A
534
)
and the extraction stage refers to Morohoshi et al.,
(2007). Serratia marcescens was grown for 15 hr on
fresh Luria-Bertani medium (1%). Production of
prodigiosin was monitored in an interval of 5 hr for
30 hr with or without the addition of bulb extracts
with concentration variants of 0.02, 0.1, 0.2 and
0.3%. Prodigiosin was extracted from cells in
acidified ethanol solution (4% 1 M HCL in ethanol).
Prodigiosin production was determined by
determining the absorbance ratio extracted at 534
nm. Percentage of prodigiosin inhibition is
calculated using following formula with a control
value of 100%:
%inhibition=
Absorbance of control-absorbance of treatment
×100%:
Absorbance of control
3 RESULTS AND DISCUSSIONS
Confirmation of QSI activity is based on none
inhibition towards growth of reference strain,
Serratia marcescens. Our results showed that none
of tested extracts inhibit the growth of S.marcescens
(OD
600
) until the end of incubation period. Control
can be seen to produce higher OD than the samples
as shown in Figure (1a and 1b)
Inhibitory Activity of Allium chinense G. Don. Extracts to Prodigiosin Synthesis by Serratia Marcescens
83

(a)
(b)
Figure 1: Effect of Bawang Batak MeOH extract at growth of S. marcescens (a), . Effect of Bawang Batak EtOAC
extract at growth of S. marcescens (b).
The results of inhibitory assay using methanolic
fractions showed inhibition at concentrations of 0.2
and 0.3%. Prodigiosin production is higher in
control than the treatments (Figure 2a). While at the
concentration of 0.02 and 0.1%, the production is
higher than the control, yet still indicating that there
was no inhibition towards prodigiosin at given
concentrations. The results from ethyl acetate
fraction (EtOAc) was lower than the control (Figure
2B).
(a)
(b)
Figure 2: Prodigiosin synthesis ; MeOH extract (a), EtOAC extract (b)
In the end of incubation period (30 hr),
methanolic fraction showed a lower prodigiosin
production than control at concentration of 0.2 and
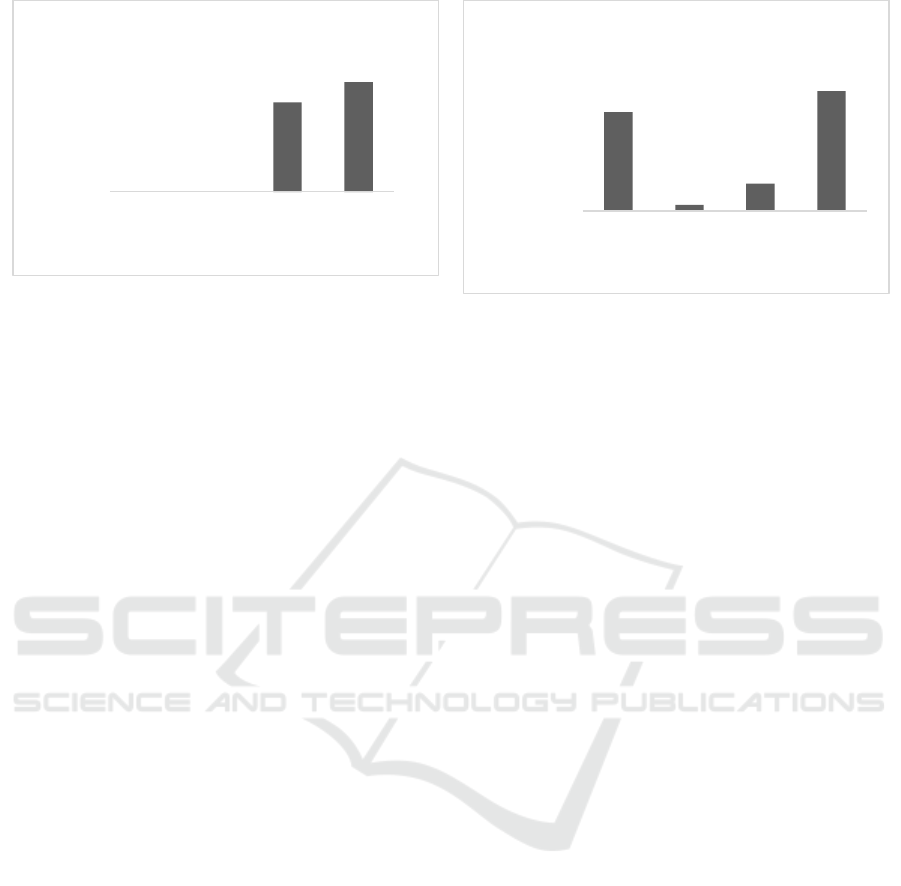
0.3% with percentage of inhibition 24.3 and 29.8%
which can be seen in Figure 3a. However, from ethyl
acetate fraction showed that all tested concentrations
inhibit the prodigiosin with the highest observed at
concentration of 0.3% with percentage of 49.2% as
shown in Figure 3b.
0.0
0.5
1.0
1.5
2.0
2.5
5 10 15 20 25 30
OD600
Incubation Time (Hour)
Methanolic Fraction
K
0,02%
0,1%
0,2%
0,3%
0.0
0.5
1.0
1.5
2.0
5 10 15 20 25 30
OD600
Incubation Time (hour)
Ethyl Acetate Fraction
Control
0,02%
0,1%
0,2%
0,3%
0.00
0.50
1.00
1.50
2.00
5 10 15 20 25 30
A534
Incubation Time (hour)
Methanolic Fraction
Control
0,02%
0,1%
0,2%
0,3%
0.00
0.10
0.20
0.30
0.40
0.50
0.60
0.70
5 10 15 20 25 30
A534
Incubation Time (hour)
Ethyl Acetate Fraction
Control
0,02%
0,1%
0,2%
0,3%
ICONART 2019 - International Conference on Natural Resources and Technology
84
(a)
(b)
Figure 2: Prodigiosin synthesis ; MeOH extract (a), EtOAC extract (b)
Our results showed that bulb extracts of
A.chinense displayed a potential array of other
bioactive properties than previously known as
antimicrobials. In this study, MeOH and EtOAc
fractions also inhibit the cell signaling
communication or quorum sensing. Although
phytochemical groups have been identified from
these bulb extracts (saponins, triterpenoids, steroids,
flavonoids, essential oils), the underlying
mechanism in displaying QSI activity is still remain
unknown (Liu et al., 2014; Jiang et al., 1999; Kuroda
et al., 1995).
In the methanol fraction, the high production of
prodigiosin from Serratia marcescens is assumed by
the effect LB medium used. Luria-Bertani medium
is a complex medium that may alter cells metabolic
pathway and thus supporting the occurrence of
quorum sensing sensing system. In a study using P.
aeruginosa, it has been shown that increased levels
of nutrients may induce the growth of bacterial
pigments (Saver et al., 2004). Luria-Bertani medium
is also likely to be a complex medium containing
signals or other factors, such as surfactants that are
needed for swarming and biofilm formation (Holden
et al., 1999). However, we are still able to document
particular inhibitory activities of extracts in certain
tested concentrations as shown in previous figures.
Both fractions showed the highest inhibitory value at
0.3% concentration to 30th (incubation period). This
is consistent with previous studies stating that, the
higher the concentration of extracts, the higher the
inhibition (Bai and Vittal., 2014; Packtiavathy et al.,
2014). In order to reveal the mechanism of QSI
activity exhibited by A.chinense bulb extracts, more
efforts are needed to support their use as potential
QSI in the future.
4 CONCLUSIONS
The results showed that Batak Onion (Allium
chinense, G. Don.) bulb extract MeOH and EtOAc
fractions were potential quorum sensing inhibitors of
Serratia marcescens without inhibiting the growth
of these bacteria.
ACKNOWLEDGEMENTS
The authors would like to express the highest
gratitudes to Ministry of Research, Technology, and
Higher Education of the Republic of Indonesia for
financially supporting us through research grant
scheme of Penelitian Tesis Magister 2019
No:11/E1/KP.PTNBH/2019 to IJ
REFERENCES
Bai, A. J., Vittal, R.R 2011. Bacterial quorum sensing and
food industry. Reviews In Food Sci and Food Safety
10: 184-194
Bai A. J., Vittal, R. R. 2014. Quorum Sensing Inhibitory
and Anti-Biofilm activity of essential oils and their in
vivo efficacy in food systems. Food Biotechnol
28:269-292
Bah, A. A., F. Wang, Z. Huang, I. H. Shamsi, Q. Zhang,
G. Jilani, S. Hussain, N. Hussain, E. Ali. 2012. Phyto-
characteristics, Cultivation and Medicinal Prospects of
Chinese Jiaotou (Allium chinense). J. Agricul & Biol.
14: 650-657
Bancirova, M. 2010. Comparison of the antioxidant
capacity and the antimicrobial activity of black and
green tea. Food Research International, 43(5), 1379-
1382
0.0
5.0
10.0
15.0
20.0
25.0
30.0
35.0
0.02% 0.1% 0.2% 0.3%
Persentage of Prodigiosin
Inhibition (%)
Concentration Bawang Batak (w/v)
Methanolic Fraction
0.0
10.0
20.0
30.0
40.0
50.0
60.0
0.02% 0.1% 0.2% 0.3%
Percentage of Prodigiosin
Inhibition (%)
Concentration Bawang Batak (w/v)
Ethyl Asetat Fraction
Inhibitory Activity of Allium chinense G. Don. Extracts to Prodigiosin Synthesis by Serratia Marcescens
85

Chai WM, Liu X, Hu YH, Feng HL, Jia YL, Guo YJ,
Zhou HT, Chen QX. 2013. Antityrosine and
antimicrobial activities of furfuryl alcohol, furfural
and furoic acid. J. BiolMacromol 57: 151155
Givskov, M., L. Olsen, and S. Molin. 1998. Cloning and
expression in Eschercia coli of the gene for
extracellular phospholipase from Serratia liquefaciens.
J. Bacteriol. 170:5855-5862
Givskov, M., J. Ostiling, L. Eberl, P.W. Lindum, A.B.
Christensen, G. Christensen, S. Molin, and S.
Kjelleberg. 1999. Two separate regulatory system
participate in control of swarming motility of Serratia
liquefaciens MG1. J. Bacteriol. 180: 742-745
Holden, M. T. G., S. R. Chahabra, R. deNys, P. stead, N.j.
Bainton, P.J. Hill, M. Manefield, N. Kumar, M.
Labbate, D. England, S.A. rice, M. Givskov, G.
Salmond, G.S. A. B. Stewart, B. W. Bycroft, S.
Kjelberg, and P. Williams. 1990. Quorum Sensing
cross talk: isolation and chemical characterisation of
cyclic dipeptides from Pseudomonas aeruginosa and
other Gram Negative bacteria. Mol. Microbiology.
33:1254-1266
Houdt RV & Michiels CW. 2010. Biofilm formation and
the food industry, a focus on the bacterial outer
surface (Review article). JAppl Microbiol. ISSN
1364-5072.
Jiang Y, Wang NL, Yao XS, Kitanaka S. 1999. Steroidal
saponin from the bulbs of Alliumchinense. Studiesin-
PlantScience. 6: 212-219. DOI: 10.1016_S0928-
3420(99)80029-9.
Kalia, V. C. (2013). Quorum sensing inhibitors: an
overview. Biotechnology advances, 31(2), 224-245.
Kim, H.-S., Lee, S.-H., Byun, Y., Park, H.-D. (2015). 6-
Gingerol reduces Pseudomonas aeruginosa biofilm
formation and virulence via quorum sensing
inhibition. Scientific reports, 5.
Kuroda M, Mimaki Y, Kameyama A, Sashida Y, Nikaido
T. 1995. Steroidal saponin from Alliumchinense and
their inhibitory activities on cyclic AMP
phosphodiesterase and Na+/ K+ ATPase. J.-
Phytochemistry. 40(4): 10711076. DOI: 0031-
9422(95)00423-8
Liu XC, Lu XN, Liu QZ, Liu ZL. 2014. Evaluation of
insecticidal activity of the essential oil of Allium -
chinense G. Don and its major constituents against
Liposcelis bostrychophila Badonnel. Journal of Asia-
Pacific Entomology. 17: 853-856. DOI: 10.1016/
j.aspen.2014.08.007
Matshumura N, Minami S, Mitsuhatshi S. Sequence of
homologous Lactamases from clinical isolates of
Serratia marcescens with different substrate
specificities. Antimikrob Agents Chemother. 1998; 41
(suppl 4): 25-41
McKay, D. L., Blumberg, J. B. (2002). The role of tea in
human health: an update. Journal of the American
College of Nutrition, 21(1), 113.
Morohoshi T, Toshitaka Shiono, Kiyomi Takidouchi,
Masashi Kato, Norihiro Kato, Junichi Kato, dan
Tsukasa Ikeda. 2007. Inhibition of Quorum Sensing in
Serratia marcescens AS-1 by Shintetic Analogs of N-
Acylomosherine Lactone. J. Appl and Envir
Michrobiol, Oct 2007. Vol 73:20.
Naibaho, Frans G, Maria B, Fachriyan HP. 2015.
Antimicrobial activity of Allium chinense G.
Don.Curr.Biochem. 2 (3): 129 – 138
Packiavathy, I.A.S.V., Agilandeswari, P., Musthafa, K.S.,
Pandian, S.K., Ravi, A.V. 2012. Antibiofilm and
quorum sensing inhibitory potential of Cuminum
cyminum and its secondary metabolite methyl eugenol
against Gram negative bacterial pathogens. Food Res.
Int. 45:85–92.
Rudrappa T., Bais H.P. 2008. Curcumin, a known
phenolic from Curcuma longa, attenuates the
virulence of Pseudomonas aeruginosa PAO1 in whole
plant and animal pathogenicity models. J.Agricul and
Food Chemist 56: 1955-1962.
Saver, K., m. C. Cullen, A. H. Rickaerd, L. A. H. Zeef,
D.G. davles, and P. Gilbert. 2004. Characterization of
nutrient induced dispersion in Pseudomonas
aureginosa PAO1 biofilm. J. bacterial. 186:7312-
7326
Sutar RL, Mane SP, Ghosh JS. Antimicrobial activity of
extract of dried kokum (Garciniaindica C). J. Food
19(3): 1207-1210.
Taga, M.E. and B.L. Blasser. 2003. Chemical
Communication Among Bacteria. Proceeding of the
National Academy of Science USA 100 (2):
1454914554
Zanatta N, Alves SH, Coelho HS, Borchhardt DM,
Machado P, Flores KM, Da Silva FM, Spader TB,
Santurio JM, Bonacorso HG, Martins MAP. 2007.
Synthesis, antimicrobial activity, and QSAR studies of
furan3-carboxamides.J .Bioorganic &Medical
Chemistry. 15(5): 1947-1958.
ICONART 2019 - International Conference on Natural Resources and Technology
86
