
Selection of Tubular Membrane Separation based on the Resistance
Performance
Lilis Sukeksi
1,2
, Che Rosmani Che Hassan
2
, Nik Meriam Sulaiman
2
and
Mohamed Kheireddine Aroua
2
1
Department of Chemical Engineering, Faculty of Engineering, Universitas Sumatera Utara, Jl. Almamater Kampus USU,
Medan 20155, Indonesia
2
Department of Chemical Engineering, Faculty of Engineering, Universiti Malaya, 50603, Kuala Lumpur, Malaysia
Keywords: Tubular Membrane, Polyphenols, Resistance, Mass Transfer and Permeate.
Abstract: Processing of fruits (such as pink guava) to produce fruit juices results in high amount of waste materials that
still contain valuable by-products (e.g. antioxidants or polyphenols). Analysis of hydrodynamic resistances
that considers gel layer formation as the main fouling mechanism and permeate flux decline was studied.
Using tubular membrane FP 100 and ES 404 performed the experiments, with a molecular weight cut-off of
100 kDa and 4 kDa respectively. Results showed that the permeate fluxes for both of the membranes increased
by increasing the Trans Membrane Pressure (TMP) and it would decrease with time. All of the resistances
increased with TMP meanwhile the mass transfer of polyphenols did not affect the TMP. All the TMP resulted
in similar fouling values for both membranes.
1 INTRODUCTION
The waste-to-wealth approach for management of the
residuals will result in the recovery of valuable by-
products as well as solving the waste disposal
problem. In the case of pink guava processing waste,
the recovery of bioactive compounds is a profitable
venture that can result in the recovery of polyphenols
and other antioxidants. Polyphenols have good
properties because they have anti-oxidant activity
properties that are beneficial for enhancing health
effects for humans (Friedman, 2002). Polyphenols
can counteract the attacks of free radicals, therefore
polyphenols can avoid the body from hereditary
diseases such as cancer and other diseases (Gökmen,
2003).
In general, polyphenol compounds can be isolated
or taken from fruit or vegetable processing wastes by
the extraction process (D’Alvise, 2000). The use of
filtration membranes for recovery, purification or
concentration of fruits and vegetables has been
extensively studied for the past 25 years. (Czekaj,
2000) identify restoration of polyphenols from pink
guava manufacture residual pulp, and these processes
can be done at low temperature; do not involve a high
energy usage and makes it possible to separate
bacterial and spore cells and to completely remove
suspended solids. However, the decrease in permeate
flux along with the increase in reaction time will
occur, this is because some material will clog the
membrane pores while others will thicken on the
membrane surface and will shape a gel. Membrane
contamination resulting in membrane fouling has
been explored by many membrane separation
researchers because it can make lower the
productivity and age of membranes. (Nilsson, 1990).
However, the decrease in flux caused by increasing
the concentration of the solution on the surface of the
membrane so that gel formation caused by complex
polyphenols substances needs to be studied further.
Furthermore, the basic mechanism so that the
occurrence of fouling which causes a decrease in flux
during the ultra filtration process of polyphenols is
still not known correctly.
Fouling membrane that occurs in membrane
filtration is influenced by three main factors, namely,
the nature of the material or membrane material used,
characteristics of the feed or sample and parameters
of the operation process. In most studies related to
ultrafiltration separation membranes (UF) a model is
made using the hydrodynamic theory based on the
formation of polarization concentrations and the
formation of solids or gels on the membrane surface,
Sukeksi, L., Hassan, C., Sulaiman, N. and Aroua, M.
Selection of Tubular Membrane Separation based on the Resistance Performance.
DOI: 10.5220/0008551501870192
In Proceedings of the International Conference on Natural Resources and Technology (ICONART 2019), pages 187-192
ISBN: 978-989-758-404-6
Copyright
c
2019 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
187

which produce hydrodynamic resistance to absorb
flow (Fane, 1987). This model can also be applied to
the process of separating polyphenols from pink
guava because this process also forms a layer of cake
and gel on the surface of the separating membrane.
Polarization of the concentration and layer of cake
can facilitate irreversible membrane contamination
by revising interactions between solvents, solutes and
membranes. Therefore, to understand the
phenomenon of the fouling process which results in
membrane process failure is by analyzing the surface
chemistry of the membrane, the interaction of solutes
to the membrane and the interaction between the
solute. So, the interaction between separation
membrane and solution can determine the occurrence
of fouling caused by adsorption of dissolved
polyphenols on the membrane surface.
The paper is part of the research that has been
done on the recovery of polyphenols from the
processing of pink guava waste (Sukeksi & Sarah,
2016), (Sukeksi et al., 2016). This paper will discuss
the effects of differences in operating pressure or
TMP on membrane fouling and permeate flux in two
types of membrane separation during the polyphenol
recovery process.
Permeate flux decline over time is the main
limiting factor that influence the membrane process.
The permeate flux decline because of feed
components increases inside the pores. This process
results in membrane fouling. The feed component
also increases on the membrane surface that result
forming concentration polarization or gel layer. Some
researchers have learned about fouling that occurs in
membranes, this is done because fouling can reduce
the productivity and lifetime of the membrane
(Nilsson, 1990). However, a decrease in flux due to
polarization and concentration of the solution
resulting in fouling, and the complex effects of
polyphenol substances need to be investigated
further.
2 MATERIAL AND METHODS
2.1 Materials
Two commercial tubular membranes with FPDF FP
200 type with nominal MWCO 200,000 and ES 404
membranes with a nominal 4,000 MWCO with a pH
operating range ranging from 1.5 - 12, and a
maximum operating pressure of 10 bar, and a
maximum operating temperature of 80 ̊ C.
Membranes are supplied and manufactured by PCI,
UK. The membrane housing used was supplied by
local supplier, with 14 mm of inner diameter and 325
mm of length, with the module configuration
contained two tubular membranes. Prior to use is
soaking overnight in 0.3% HNO
3
to eliminate
impurities left from the mechanized process or
additives used for stabilization washes the
membranes. Membrane equipment modules used for
polyphenol recovery from pink guava processing
waste consist of one diaphragm pump, 10 liter
capacity feed reservoir, permeate collection reservoir,
two inlet and outlet pressure gauges, valves for
control and balance pressure and equipped with
monitors for data processing. The all material of
equipment such as, pump, feed reservoir and all
connection tubing are used material base of stainless
steel. Folin-Ciucalteu and Gallic acid were from
Sigma-Aldrich (Germany) and Sodium Carbonate
powder, Nitric Acid is supplied by Fluka (Germany).
Processing of pink guava waste is collected from
Sitiawan Perak, which is produced from a Decanter
separator and Refiner separator with a composition of
50%. If this pink guava waste is stored in an improper
manner it will result in a rapid loss of polyphenols, so
the extraction process cannot be carried out.
Therefore, the waste must be stored properly in the
refrigerator to prevent fungal growth and oxidation.
2.2 Methods
2.2.1 Extract Preparation
Extraction methods using solvents are the most
common way to isolate a compound from various
fruits, as well as vegetables, such as polyphenol
compounds. To isolate the substance in the extract is
very dependent on the type of solvent used, because
each type of polyphenols compound has a different
polarity. Waste pink guava processing extract for
total polyphenols content analyses are prepared by
following method of Swain and Hillis (1959), with
some modifications. Base in our study before, the best
solvent for extraction to recovery polyphenol from
pink guava wastes processing are Methanol/Water at
60% and the second is water. The best composition
ratio between the sample wastes and solvent is 1:40.
In this project the polyphenols within the pink guava
wastes processing is extracted using water as a
solvent. The choice of water as a solvent is based on
the information that water more saves for human than
organic solvent. Solid pink guava waste and water are
then stirred using a blender constantly for 10 minutes
until a homogeneous slurry or solution is produced.
After 12 hours, the aqueous extract is separated from
the solid by removed the upper of solution to reduce
ICONART 2019 - International Conference on Natural Resources and Technology
188

the suspended solid content. The clear solution
produced will be used for the recovery process of
polyphenols using FP 200 and ES 404 membrane UF.
2.2.2 Recovery Polyphenols
Membrane separation is an alternative method for the
solvent separation process from polyphenol extract.
The system consists of PVDF 200 FP and ES 404
membrane connected to a feed reservoir and a
diaphragm pump. The steps involve in experiment
are:
To determine the flux of water by entering tap
water into the membrane by turning on the pump at a
certain TMP and calculating the volume of permeate
(Vp) generated at a certain time (t) passing through
the surface area of the separation membrane (A) using
the equation below:
J =
First Cleaning.
The cleaning involved by initial water flushing for ten
minutes and nitric acid 0.3% 30 minutes followed by
water flushing again for ten minutes, to remove
impurities left from the mechanized process or
additives used for stabilization. The end of water
flushing flux was measured by using data storing
from the data lodging.
Ultra Filtration.
In operation, the feed stream extract of pink guava
processing wastes is pumped using a diaphragm
pump through the both of tubular PVDF membrane.
The process of ultra filtration tubular membranes
begins with the permeate port being closed, this is to
allow cross speed before permeating out, with both
inlet valves for feed solution and retentate solution in
wide open conditions. After the pump is run, the valve
for the inlet channel is opened and the valve for the
solution on the retentate is slowly closed to produce
the preferred Trans Membrane Pressure (TMP). With
the increase in volume of solution at the permeate, the
concentration of polyphenols will also increase. Ultra
filtration experiment is carried out with continuous
retentate recycling. The result permeate is
continuously removed, until the desired volume
concentration ratio (VCR) is achieved.
VCR =
Where:
VCR or (Volume of Concentration Ratio),
V
f
(m
3
) is initial volume of the feed
V
R
(m
3
) is retentate volume
All data is collected and record by computer via a
data logger. The samples that are resulted from
permeate and retentate are provided to analyze total
polyphenols content.
Second Cleaning.
The cleaning procedure is the same with the first
cleaning procedure before ultra filtration processing,
and the water flushing flux was also measured by
using data storing from the data lodging.
All the processing procedures were repeated for
three times, by using the same membrane which are,
each step operation until VCR = 4 were reached and
at TMP = 1, 2 and 3 bar, respectively.
Determination of Total Polyphenols Content.
The total polyphenol content produced was
determined in all samples using the Folin-Ciocalteau
method, which was modified by the theory of
(Singleton, 1965) with some modifications. Gallic
acid calibration standard solution is prepared for
0.01-0.1 mg/ml by accurately weighing and
dissolving with of distilled water as a solvent. The
solution mixture consisted of 200 l extract of pink
guava waste sample mixed with 1.5 ml of Folin-
Ciocalteau reagent and left at room temperature for 5
minutes then 1.5 ml of sodium bicarbonate solution
was added to the mixture. After standing 90 minute at
room temperature, absorbance is measured at 760 nm.
The results obtained are expressed as mg / ml Gallic
equivalent (GAE). The gallic acid standard curve can
be seen in Figure 1 which is in the range of Gallic acid
concentration from 0.025 to 0.1 mg / ml, and this will
be used to make a calibration curve.
Calculation of Membrane Performance.
The membrane performance was measured by
concentration factor (C
R,P
) and recovery (C
R,P
) of
polyphenols content. Concentration factor is the
concentrations of polyphenols in either permeate or
retentate solution divided by its concentration in the
feed solution.
R
(R,P)
(%) =
Selection of Tubular Membrane Separation based on the Resistance Performance
189
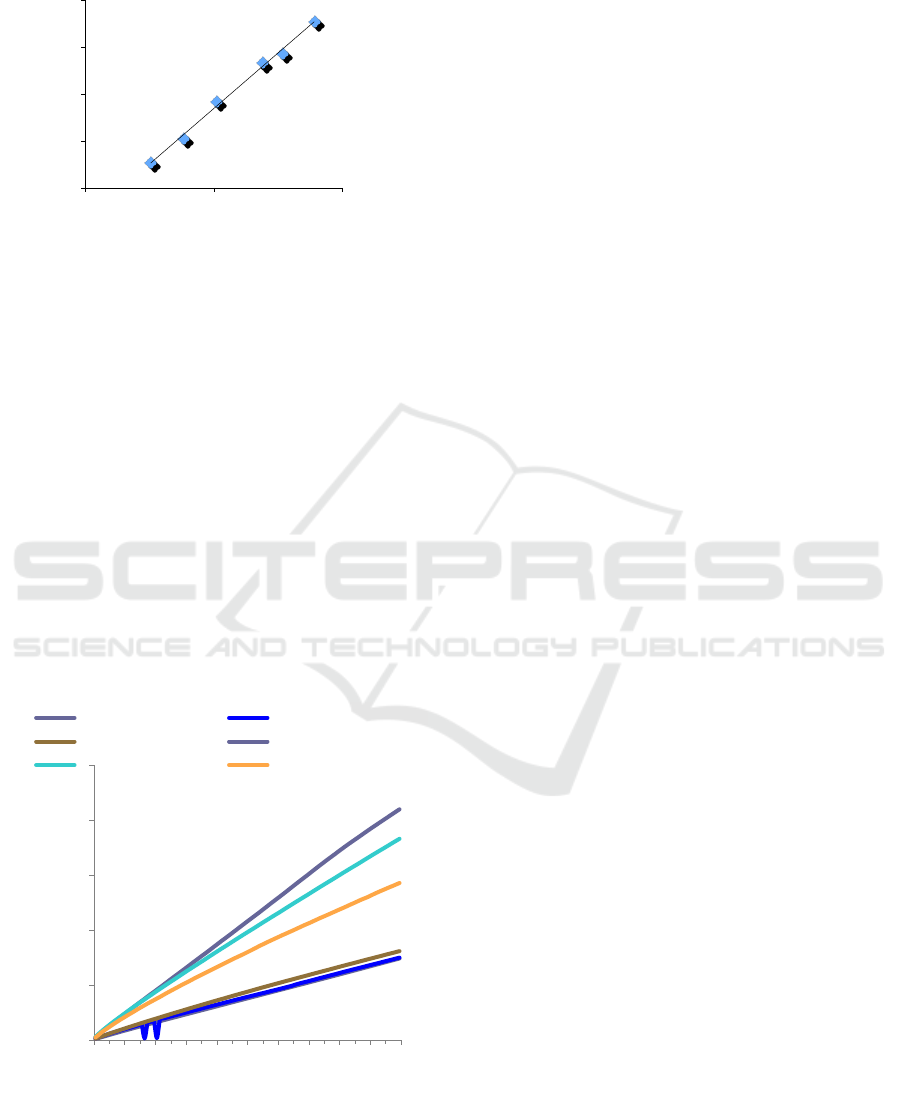
Figure 1: Gallic acid standard curve based on the data
collected at absorbance 760 nm.
3 RESULTS AND DISCUSSIONS
3.1 Influence of TMP on the Permeate
Volume
The increasing permeates volume as a function of
time for tubular membrane at different pressures is
shown in Figure 2. The permeate volume collected
during recovery of pink guava processing wastes
increased with time but at a decreasing rate. For
membrane ES 404, the increasing of permeate
volume for all transmembrane pressure are similar
trend, there is no significant different between them.
Figure 2: Permeate volume vs time for recovery
polyphenols using PVDF membrane FP 200 and ES 404 at
different TMP.
But for the membrane FP 100 the permeate volume
collected at any time decrease with TMP increase.
The lower permeation rates for pink guava processing
wastes in comparison with those of water were due to
membrane fouling.
For the pure water, the permeate volume collected
during ultra filtration increase linearly with time for
both of the membrane. In these cases, total resistance
(R
t
)
and membrane resistance (R
m
)
constant
throughout the whole operation and J or flux also
constant at TMP constant.
3.2 Influence of TMP on The Permeate
Flux
Investigational data related to the permeate flux
decline for both of the membrane are presented in
Figure 3.
The initial flux decline was 20-37% and 17-29%
of the total flux decline for membrane FP 100 and ES
404, respectively at TMP 1-3 Bar.
The steady state was established after 20 minute
of operation and the steady state permeate fluxes were
60-80% and 70-80% of their initial values for
membrane FP 100 and ES 404, respectively.
Note that the initial flux of the permeate does not
depend on the speed of the feed flow in the tubular
membrane. This can be explained by the fact that at
the start of the separation process, permeate flux is
caused more by fouling that occurs on the internal
membrane, which is clearly not significantly affected
by the feed flow rate. Generally, the permeate flux
increases initially with the transmembrane or TMP
pressure applied, and then the flux will decrease with
increasing pressure from the transmembrane.
Contrary to this, the permeate flux for FP 100
membrane decreased with increased the TMP and for
ES 404 all the permeate flux for different TMP has
similar value.
The flux will reach steady state condition when
the cake layer has grown to the equilibrium thickness.
From the Figure 4.19 the steady state of flux
decreased with the increase of TMP. (Song, 1998),
already assumed the equilibrium thickness of cake
layer increase with the applied pressure. It is because
a thicker cake layer is needed to absorb a higher
pressure.
Cassano et al. (2008) found the effect of TMP on
the steady state of permeate flux which show a linear
increase with TMP at lower pressures. Meanwhile at
higher pressures the permeate fluxes approach a
limiting value independent of further increases in
TMP. Their point was considering the pressure
independence as the peak of TMP (100 kPa). The first
y = 4,7354x - 0,0676
R² = 0,9953
0,00
0,10
0,20
0,30
0,40
0,00 0,05 0,10
Absorbance (nm)
Concentration gallic acid (mg/ml)
0
500
1000
1500
2000
2500
1 31 61 91
Permeate volume (ml)
Time (minutes)
TMP = 1 Bar ES 404 TMP = 2 Bar ES 404
TMP = 3 Bar ES 404 TMP = 1 Bar FP 200
TMP = 2 Bar FP 200 TMP = 3 Bar FP 200
ICONART 2019 - International Conference on Natural Resources and Technology
190

fluxes decline at their first domain and was 75.8-
89.1% of the total flux decline. The steady state value
of flux shown after 34-84 minutes of processing and
steady state of permeate fluxes were 22-43% from
their first value.
Figure 3: Permeate Flux Decline Vs Time.
3.3 Hydraulic Permeability and
Membrane Resistance
The hydraulic permeability of the new clean
membrane was 114.9 L/m2hBar for FP 100
membrane; meanwhile for ES 404 membrane was
70.8 L/m2hBar at 25ºC. New membrane resistance,
R
m
, was calculated from Eq. (3) to be 3.5 x 10
12
m
-1
forFP 100 membrane and 5.7 x 10
12
m
-1
for ES 404
membrane respectively. The membrane resistance
calculated after the cleaning procedure was reported
to its original value as observed from the hydraulic
permeability data.
3.4 Influence of Transmembrane
Pressure on the Total Resistance
As shown in Figure 4 the total resistance (R
t
)
increased with increased the TMP for both of the
membrane. This phenomenon can be explained by
assuming that an increase of pressure improved flux
and convective flow of the solute towards the
membrane. Therefore, the concentration polarization
was more evident determining an increase of fouling
resistance.
Figure 4: The influence of TMP on total resistance FP 100
membrane and ES 404 membrane.
4 CONCLUSIONS
Permeate volume increase with time for both of
membrane ES 404 and membrane FP 200. For
membrane ES 404, the increasing permeate volume
for all TMP had a similar trend but for membrane FP
200 increasing of TMP resulted in increases of
permeate volume. Initial flux decline was achieved
20% for membrane FP 200 and 37% for membrane
ES 404 from the total initial flux at TMP 1-3 bar and
steady state settled after 20 minutes of processing for
both of membranes. The hydraulic permeability of the
new clean membrane was 114.9 L/m2hbar for FP 200
membrane. Meanwhile for ES 404 membrane was
70.8 L/m2hbar at 25ºC. New membrane resistance,
R
m
, was 3.5 x 10
12
m
-1
for membrane FP 200 and 5.7
x 10
12
m
-1
for ES 404. Total resistance (R
t
) increased
with increased of TMP for both of the membrane.
Meanwhile the R
2
for membrane FP 200 is 0.9719
and for membrane ES 404 is 0.9949.
REFERENCES
Cassano, A., Mcchia, A. and Drioli, E. (2008). Analyses of
hydrodynamic resistances and operating parameters in
0
10
20
30
40
50
60
70
80
90
100
0 50 100
Permeate Flux (l/m2hr)
Time (minutes)
FP 200, TMP = 1 bar FP 200, TMP = 2 bar
FP 200, TMP = 3 bar ES 404, TMP = 1 bar
ES 404, TMP = 2 bar ES 404, TMP = 1 bar
y = 114,9x + 104,3
R² = 0,9683
y = 65,5x + 4,1
R² = 0,9839
0
100
200
300
400
500
600
700
800
0 2 4 6
Flux (L/m2h)
TMP (bar)
Membrane FP 200 Membrane ES 404
Selection of Tubular Membrane Separation based on the Resistance Performance
191

the ultrafiltration of grape must. Journal of Foof
Engineering, 171-177.
Czekaj, P., F., Lopez, and Guell, C. (2000). Membrane
fouling during microfiltration of fermented beverage.
Journal of Membrane Science, 199-212.
D'Alvise, N., Lesuerur-Lambert. C., Fertin, B., Dhulster, P.
& Gillochon, D. (2000). Removal of polyphenols and
recovery of proteins from alfalfa white protein
concentrate by ultrafiltration and adsorbent resin
separation. Separation Science and Technology, 35,
2453-2472.
Fane, A. G., Fell, C., J., D. (1987). A review of fouling and
fouling control in ultrafiltration. Desalination, 117-136.
Friedman, M., Jurgens, H., S. (2002). Effect of pH on the
stability of plants phenolic compounds. Journal of
Agricultural and Food Chemistry, 48, 2101-2110.
Gokmen, V. A. (2003). Influence of conventional
clarification and ultrafiltration on the phenolic
composition of golden delicious apple juice. Journal of
Food Quality, 26, 257-266.
Nilsson, S., I. (1990). Protein fouling of UF membrane:
causes and consequences. Journal of Membrane
Science, 56, 121-142.
Singleton, V. L. (1965). Colorimetry of Total Phenolics
with Phosphomolybdic-Phosphotungstic Acid
Reagents. Amer. J. Enol. Viticult, 16, 144-158.
Song, L. (1998). Flux Decline in Crossflow Microfiltration
and Ultrafiltration: Mechanisms and Modelling of
Membrane Fouling. Journal of Membrane Science,
139, 183-200.
Sukeksi, L., Che Rosmani, C., H., Nik Meriam, N., S.,
Rashidi, H., Emami, S., D. (2016). Polyphenols
Recovery from Tropical Fruits (Pink Guava) Waste via
Ultrafiltration Membrane Technology Application by
Optimum Solvent Selection. Iranian Journal of
Chemistry and Chemical Engineering (IJCCE), 35 (3),
53-63.
Sukeksi, L., Sarah, M. (2016). Characterizations and
Extraction of Polyphenols from Residual Pulp of Pink
Guava as Source of Antioxidants. Journal of
Engineering Applied Science, 11, 5209-5216.
ICONART 2019 - International Conference on Natural Resources and Technology
192
