
Formation Process of Graphene Nano Sheets
Herlince Sihotang
1
, Rikson Siburian
1,2*
, Crystina Simanjuntak
3
, Saur Lumban Raja
1
, Minto Supeno
1
,
Vivi Sukmawati
1
and Zul Alfian
1
1
Department of Chemistry, Faculty of Mathematics and Natural Sciences, Universitas Sumatera Utara,
Jl. Bioteknologi No. 1, Medan 20155, Indonesia
2
Nano Medicine Center, Universitas Sumatera Utara, Medan, Indonesia
3
Postgraduate Chemistry Study Program, Faculty of Mathematics and Natural Sciences, Universitas Sumatera Utara,
Jl. Bioteknologi No. 1 Kampus USU, Medan, Indonesia
vsukmawati81@gmail.com, zulalfian16@yahoo.com
Keywords: Graphene Nano Sheets, Magnesium, Epoxide, FTIR, Hummer Method.
Abstract: Formation process of Graphene Nano Sheets (GNS) was described in this paper. The aims of this paper are
to synthesize of GNS and to propose formation process of GNS base on magnesium (Mg) as a reductor
agent. This research is an experiment laboratory research. The modification of Hummer’s method was
chosen to generate GNS. Then, GNS was characterized with FTIR. The results data show that Mg may be
used to reduce epoxide functional groups.
1 INTRODUCTION
Graphene may be applied to many applications
(Geim, 2007; Xu, 2015; Novoselov, 2004; Terrones,
2010; Soldano, 2010). Unfortunately, it cannot be
produced naturally. It is possible to synthesize base
on graphite as a raw material (Choi, 2011; Bhuyan,
2016; Lee, 2016). Generally, GNS may be
synthesized by using CVD (Wang, 2009; Juang,
2010; Bárcenas, 2018; Jacobberger, 2015) and
chemical method (Siburian, 2012, 2013, 2014, 2017;
Ratih, 2018; Supeno, 2018; Sebayang, 2018). GNS
may be generated with facile method. Commonly,
chemical method in term of producing graphene uses
graphite as a raw material (Bhuyan, 2016; Eigler,
2013; Saleem, 2018; Dimiev, 2014). Chemically
method may be expected to large scale graphene
production (Kairi, 2018; Li, 2014; Zhong, 2015;
Parvez, 2015; Park, 2009), and exfoliated graphite
(Gao, 2017). The commercialization of graphene
and its derivatives product will be visible if it may
produce on low cost, best quality, large scale and
green material (Tatarova, 2017; Zhong, 2015).
Therefore, in this paper the formation of GNS base
on chemically method was studied, thereby large
scale production of GNS may be done in future.
2 MATERIALS AND METHODS
2.1 Synthesis of GNS
In this research, GNS was produced with Hummer’s
modified method (Siburian, 2012). Briefly, 1 gram
graphite was mixed with 75 mL H
2
SO
4
96% and 1
gram NaNO
3
, stirred for 4 hours. Then, 5 gram
KMnO
4
gradually was added into solution, stirred 4
hours, T = 20
o
C at ice water bath condition. At the
end of 4 hours, the solution was move from ice
water bath and it was continue stirred for 48 hours at
room temperature to form dark brown solution.
Subsequently, 5 mL H
2
O
2
30% and 100 mL H
2
SO
4
5% was put into solution, respectively, stirred 2
hours to form graphite oxide. After that, graphite
oxide solution was centrifuged at 6500 rotor per
minute (rpm) for 20 minutes to separate between
residue and supernatant. Then, residue was added 10
mL piranha solution and aquades, centrifuged at
6500 rpm for 20 minutes, respectively to form
graphite oxide solution.
100 mL graphite oxide solution was
ultrasonicated on 5060 Hz for 5 hours to form
graphene oxide. Finally, 10 mL graphene oxide
solution was added 0.01 mg magnesium (Mg)
powder, stirred for 72 hours, filtrated and heated at T
= 80 ºC for 24 hours to produce GNS. Graphite,
36
Sihotang, H., Siburian, R., Simanjuntak, C., Raja, S., Supeno, M., Sukmawati, V. and Alfian, Z.
Formation Process of Graphene Nano Sheets.
DOI: 10.5220/0008839400360038
In Proceedings of the 1st International Conference on Chemical Science and Technology Innovation (ICOCSTI 2019), pages 36-38
ISBN: 978-989-758-415-2
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
graphite oxide, graphene oxide and GNS were
characterized by using FTIR, respectively.
3 RESULTS AND DISCUSSION
First of all, graphite as a raw material was
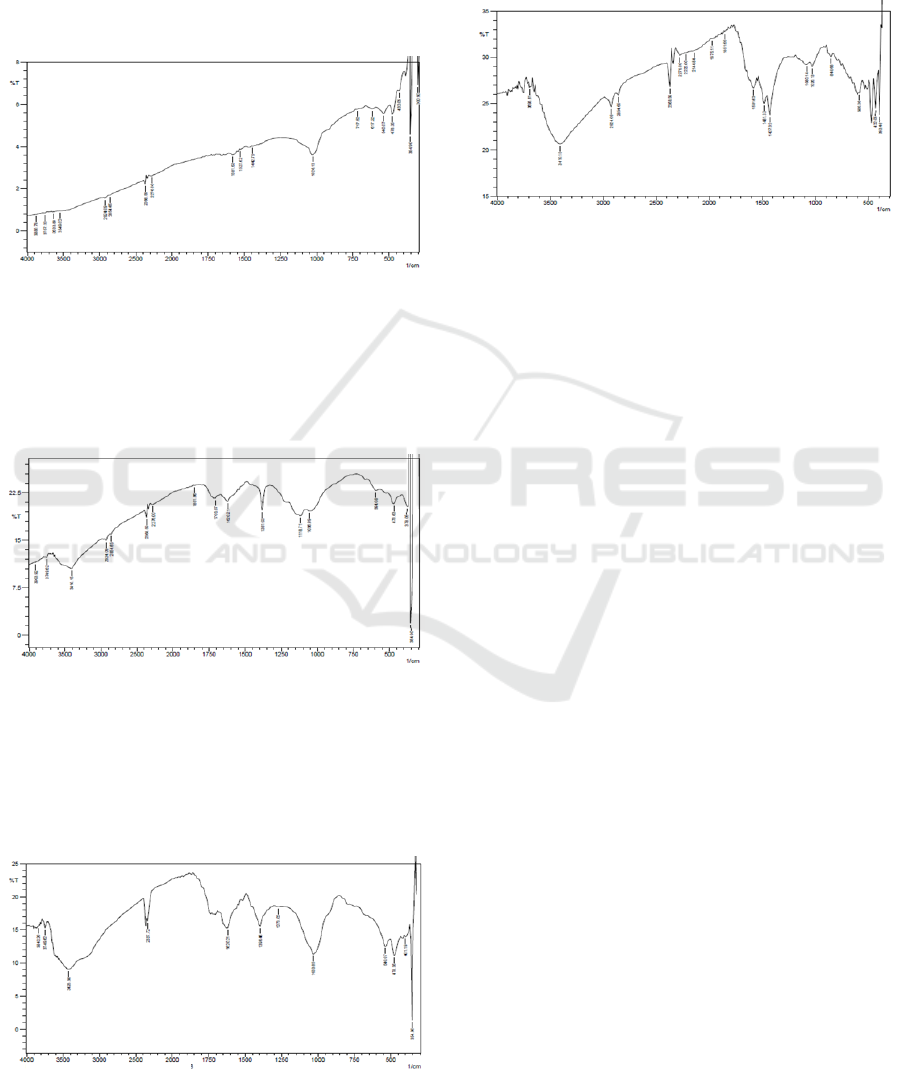
characterized with FTIR (Figure 1).
Figure 1: FTIR spectrum of graphite.
Figure 1 show that there is a weak and broad peak at
1581 cm
-1
, indicating graphite consists of double
bond aromatic carbon. In contrast, FTIR spectra of
graphite oxide is totally different compare to
graphite (Figure 2).
Figure 2: FTIR spectrum of graphite oxide.
FTIR data indicates that graphite oxide has hydroxyl
functional group (-OH) (wave number (υ) = 3,410 cm
-
1
), CH
2
(2,924 cm
-1
), CH
3
(2,854 cm
-1
), C=O
(1,705
cm
-1
), and C-O
(1,118 cm
-1
). Meanwhile, FTIR data of
graphite oxide may be seen in Figure 3.
Figure 3: FTIR spectrum of graphene oxide.
The formation of graphene oxide may be indicated
with υ = 3,410 cm
-1
(-OH), 1,620 cm
-1
(C=O), and
1,273 cm
-1
(C-O). Finally, graphene oxide was
reduced by using Mg to form GNS (Figure 4). In the
presence of Mg, the epoxide functional group may
be replaced by Mg.
Figure 4: FTIR spectrum of graphene.
4 CONCLUSIONS
The formation of graphene occurs when functional
group of graphene oxide was reduced by Mg. Mg
may only reduced epoxy functional group.
REFERENCES
Geim, A. K., Novoselov, K. S., The rise of graphene,
Nature Materials, 2007, 6, 183–191.
Xu, Y., Shi, G., Duan, X., Self-Assembled Three-
Dimensional Graphene Macrostructures: Synthesis
and Applications in Supercapacitors, Acc. Chem. Res.,
.2015, 48, 6, 1666-1675.
Novoselov, K. S., Geim, A. K., Morozov, S. V., Jiang, D.,
Zhang, Y., Dubonos, S. V., Grigorieva, I. V., Firsov,
A. A., Electric Field Effect in Atomically Thin Carbon
Films, Science, 2004, 306 (5696), 666-669.
Terrones, M., Botello-Méndez, A. R., Campos-Delgadoc,
J., López-Urías, F., Vega-Cantúd, Y. I., Rodríguez-
Macías, F. J., Elías, A. L., Munoz-Sandoval, E., Cano-
Márquez, A. G., Charlier, J. C., Terrones, H.,
Graphene and graphite nanoribbons: Morphology,
properties, synthesis, defects and applications, Nano
Today, 2010, 5, 351—372.
Soldano, C., Mahmood, A., Dujardin, E., Production,
properties and potential of graphene, Carbon, 2010, 48
(8), 2127-2150.
Wang, X., You, H., Liu, F., Li, M., Wan, L., Li, S., Li,
Q., Xu, Y., Tian, R., Yu, Z., Xiang, D., and Cheng,
J., Large‐Scale Synthesis of Few‐Layered Graphene
using CVD, Chem. Vap. Deposition 2009, 15, 53–56.
Juang, Z,Y., Wu, C. Y., Lu, A. Y., Su, C. Y., Leou, K. C.,
Chen, F. R., and Tsai, C. H., Graphene synthesis by
chemical vapor deposition and transfer by a roll-to-roll
process, Carbon 48 (2010) 3169 – 3174.
Formation Process of Graphene Nano Sheets
37

Bárcenas, A. M., Perez-Robles, J. F., Vorobiev, Y. V.,
Ornelas-Soto, N., Mexicano, A. and García, A. G.,
Graphene Synthesis Using a CVD Reactor and a
Discontinuous Feed of Gas Precursor at Atmospheric
Pressure, Journal of Nanomaterials, 2018, Article ID
3457263, 1-12.
Jacobberger, R. M., Machhi, R., Wroblewski, J., Taylor,
B., Gillian-Daniel, A. L., and Arnold, M. S., Simple
Graphene Synthesis via Chemical Vapor Deposition,
J. Chem. Educ., 2015, 92, 11, 1903-1907.
Bhuyan, Md. S. A., Uddin, Md. N., Islam, Md. M.,
Bipasha, F. A., and Hossain, S. S., Synthesis of
graphene, International Nano Letters, 2016, 6, 2, 65–
83.
Lee, H. C., Liu., W. W., Chai, S. P., Mohamed, A. R., Lai,
C. W., Khe, C. S., Voon, C. H., Hashim, U., Hidayah,
N. M. S., Synthesis of Single-layer Graphene: A
Review of Recent Development, Procedia Chemistry,
2016, 19, 916-921.
Siburian, R., and Nakamura, J., Formation Process of Pt
Subnano-Clusters on Graphene Nanosheets, J. Phys.
Chem. C, 2012, 116, 43, 22947-22953.
Siburian, R., Kondo, T., and Nakamura, J., Size Control to
a Sub-Nanometer Scale in Platinum Catalysts on
Graphene, J. Phys. Chem. C., 2013, 117, 7, 3635-
3645.
Siburian, R., Support Material Effect for Pt Catalytic
Activity at Cathode, International Research Journal of
Pure and Applied Chemistry, 2014, 4, 5, 541-550.
Siburian, R., Sebayang, K., Supeno, M. and Marpaung, H,
Effect of Platinum loading on Graphene Nano Sheets
at Cathode, Orient. J. Chem., 2017, 33, 1, 134-140.
Supeno, M., and Siburian, R., New route: Convertion of
coconut shell tobe graphite and graphene nano sheets,
Journal of King Saud University – Science, 2018,
https://doi.org/10.1016/j.jksus.2018.04.016
Sebayang, K., Siburian, R., and Supeno, M., Graphene
Nanosheets Effect to Improve CO-Tolerance of
Pt/Graphene Nanosheets Catalyst, Orient. J. Chem.,
2018, 34, 6, 2814-2818.
Ratih, D., Siburian, R., and Andriayani, The performance
of graphite/N-graphene and graphene/N-graphene as
electrode in primary cell batteries, Rasayan J. Chem.,
2018, 11, 4, 1649-1656.
Bhuyan, Md. S. A., Uddin, Md. N., Islam, Md. M.,
Bipasha, F. A., Hossain, S. S., Synthesis of graphene,
International Nano Letters, 2016, 6 (2), 65–83.
Eigler, S., Heim, M. E., Grimm, S., Hofmann, P., Kroener,
W., Geworski, A., Dotzer, C., Röckert, M., Xiao, J.,
Papp, C., Lytken, O., Steinrück, H. P., Müller, P.,
Hirsch, A., Wet Chemical Synthesis of Graphene,
Advanced Materials, 2013, 25 (26), 3583-3587.
Saleem, H., Haneef, M., Abbasi, H. Y., Synthesis route of
reduced graphene oxide via thermal reduction of
chemically exfoliated graphene oxide, Materials
Chemistry and Physics, 2018, 204, 1-7.
Dimiev, A. M., Tour, J. M., Mechanism of Graphene
Oxide Formation, ACS Nano, 2014, 8 (3), 3060-3068.
Kairi, M. I., Dayou, S., Kairi, N. I., Bakar, S. A., Vigolo
B., Mohamed, A. R., Toward high production of
graphene flakes – a review on recent developments in
their synthesis methods and scalability, J. Mater.
Chem. A, 2018, 6, 15010-15026.
Li, Y., Chopra, N., Progress in Large-Scale Production of
Graphene. Part 1: Chemical Methods, Journal of the
Minerals, Metals & Materials Society, 2014, 67 (1),
34-43.
Zhong, Y. L., Tian, Z., Simon, G. P., Li, D., Scalable
production of graphene via wet chemistry: progress
and challenges, Materials Today, 2015, 18 (2), 73-78.
Parvez, K., Yang, S., Feng, X., Müllen, K., Exfoliation of
graphene via wet chemical routes, Synthetic Metals,
2015, 210, 123–132.
Park, S., Ruoff, R. S., Chemical methods for the
production of graphenes, Nature Nanotechnology,
2009, 4, 217–224.
Gao H., Zhu K., Hu, G., Xue, C., Large-scale graphene
production by ultrasound-assisted exfoliation of
natural graphite in supercritical CO
2
/H
2
O medium,
Chemical Engineering Journal, 2017, 308, 872–879.
Tatarova, E., Dias, A., Henriques, J., Abrashev, M.,
Bundaleska, N., Kovacevic, E., Bundaleski, N.,
Cvelbar, U., Valcheva, E., Arnaudov, B., Botelho do
Rego, A. M., Ferraria, A. M., Berndt, J., Felizardo, E.,
Teodoro, O. M. N. D., Strunskus, Th., Alves, L. L.
Gonçalves, B., Towards large-scale in free-standing
graphene and N-graphene sheets, Sci Rep., 2017, 7,
10175.
ICOCSTI 2019 - International Conference on Chemical Science and Technology Innovation
38
