
Synthesis and Characterization of Superabsorbent Polymer based on
Carboxymethyl Cellulose, Breadfruit Starch and Aluminum Sulfate
Darwin Yunus Nasution*, Marpongahtun, Dede Ibrahim Muthawali, A. D. Budiman and Zulfikar
Departement of Chemistry, Faculty of Mathematics and Natural Sciences, Universitas Sumatera Utara, Medan, Indonesia
zulfikarsimatupang47@gmail.com
Keywords: Superabsorbent Polymer, Carboxymethyl Cellulose, Breadfruit Starch.
Abstract: Superabsorbent polymer is a polymeric material that able to absorb a large amount of water. The purpose of
this study is to synthesize and measure water absorption capacity and study the crosslinking process of
superabsorbent-aluminum-breadfruit starch (Al-CMC-BS). The preparation of Al-CMC-BS was done in two
steps. The first step was reacting CMC with aluminum sulfate so that the aluminum-carboxymethyl
cellulose (Al-CMC) film was produced and then mashed into powder. Al-CMC powder was dissolved in
water and reacted with BS solution to obtain Al-CMC-BS. Furthermore, Al-CMC-BS produced was
determined their water absorption capacity, morphology with SEM, functional group with FTIR and
transition glass temperature with DSC. The results showed that the absorption capacity of water from Al-
CMC-BS reached 2,444.44 %. SEM analysis shows the formation of a more homogeneous Al-CMC-BS
mixture than before mixing. The FTIR spectrum shows the formation of crosslink between Al-CMC and
BS. DSC analysis shows that there is one Tg value of Al-CMC-BS that is on 95.85˚C, which is in between
Tg of BS on 118.72˚C and Tg of CMC on 94.23˚C. This shows that the mixture of Al, CMC and BS is
miscible mixture
1 INTRODUCTION
Polymer superabsorbent (SAP) is the most
interesting study in modern polymer technology
because of its ability to absorb water up to 550 g of
water per gram of dry SAP polymer (Klinpituksa
and Kosaiyakanon, 2017). This ability to absorb
high water causes SAP to be widely used in various
fields of application such as concrete additives
(Mechtcherine, 2016) health supplies, medical
materials (Sadeghi and Soleimani, 2013), sewage
treatment and agriculture(Salavati et al., 2018; Tao
et al., 2018). Essentially SAP is a three-dimensional
crosslinked network polymer that has hydrophilic
characteristics and is not soluble in water. The
hydrophilic nature is caused by the presence of ionic
function groups such as carboxylic and hydroxyl
groups found along the polymer chain so that it
pushes to draw diffuse water into the network (Raju
et al., 2003). The molecular structure of SAP is a
three-dimensional cross link network that experience
swelling and insoluble by solvating water molecules
through the formation of hydrogen bonds. Water
absorption also causes a decrease in SAP entropy,
making it able to experience swelling daninsoluble
(Abdel-raouf, 2019).
Polyacrylate, a synthetic polymer, is the main
base material used in the industry to make SAP.
Polyacrylate is modified into network molecules
using organic crosslinking agents and initiators so
that crosslinked SAP is formed. Polyacrylate is
made from an acrylic acid polymerization reaction.
Acrylic acid is a byproduct in the process of making
ethylene and fuel oil. Therefore, polyacrylate is a
non-renewable material that relies on the
petrochemical industry. In addition, the absorption
of water is still relatively lower compared to SAP
based on natural materials (Chatterjee, 2002).
Lately, it was developed preparation of
biopolymer-based SAPs derived from agricultural
products such as corn starch, cassava starch, sago
starch and cellulose and its derivatives (Chandra
Sutradhar et al., 2015), (Weerawarna, 2009).
Compared to petroleum-based polymers,
biopolymers have advantages due to high
hydrophilicity, renewable, biodegradable and non-
toxic (Weerawarna, 2009), (Nnadi and Brave, 2011).
116
Nasution, D., Marpongahtun, ., Muthawali, D., Budiman, A. and Zulfikar, .
Synthesis and Characterization of Superabsorbent Polymer based on Carboxymethyl Cellulose, Breadfruit Starch and Aluminum Sulfate.
DOI: 10.5220/0008857101160120
In Proceedings of the 1st International Conference on Chemical Science and Technology Innovation (ICOCSTI 2019), pages 116-120
ISBN: 978-989-758-415-2
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

In this study, Al-CMC-BS was synthesized from
carboxymethyl cellulose (CMC), breadfruit starch
(BS) isolated from breadfruit and aluminum sulfate
octadecahydrate as crosslinking agents. Breadfruit is
obtained from breadfruit plants where breadfruit has
a starch content of 19.09 % (Masita, 2017).
Breadfruit plants grow a lot in Indonesia, especially
North Sumatra Province.
The purpose of this study was to synthesize and
characterize superabsorbent polymer based on CMC
and breadfruit starch. The resulting Al-CMC-BS was
characterized by measuring water absorption
capacity, FTIR spectrum, morphology with SEM,
glass transition temperature (Tg) with DSC.
2 MATERIALS AND METHODS
2.1 Materials
Breadfruit starch is isolated from ripe breadfruit.
Chemical used, which is sodium carboxymethyl
cellulose and aluminum sulfate octadecahydrate are
purchased from Merck & Co.
2.2 Methods
2.2.1 Isolation of Starch from Breadfruit
Ripe breadfruit is washed with water, peeled, cut
into smaller sizes and then ground. Furthermore,
breadfruit that has been finely filtered to separate the
pulp. Filtrate is left for 24 hours and separated by
decantation. The precipitate (BS) is dried at 70˚C.
2.2.2 Preparation of the Croos-Linked
Al-CMC
About 2.8 g of sodium carboxymethyl cellulose was
dissolved with 100 mL of distilled water, heated on
a hot plate for 1 hour at 70˚C, then added 0.02 g
octadecahydrate cross-linker and stirring continued
for 30 minutes again. The formed solution pour over
the Teflon pan and dry it at 80˚C. The film formed is
then finely ground and the water absorption capacity
(WAC) is determined.
2.2.3 Preparation of Cross-Linked
Al-CMC-BS
Breadfruit starch as much as 0.5 g was dissolved
with aquadest at 80˚C while stirring for 45 minutes
until gelatin was formed, then added 0.5 g Al-CMC
powder while stirring with a magnetic stirrer at 70˚C
for 30 minutes. The mixture formed is then poured
on a mold pan, then dried for 12 hours at 105˚C.
Solids formed are used for examination of WAC,
SEM, FTIR and DSC.
2.3 Characterization
2.3.1 Water Absorption Capacity (WAC)
The WAC of the sample is determined by inserting
the sample into a tea bag, then soaking it in distilled
water for 24 hours and then weighing it down. The
WAC price is calculated using equation:
WAC (%) =
1
21
W
WW −
(1)
W1: Weight of sample after soaking
W2: Weight of dry sample
2.3.2 Fourier Transform Infra-Red (FT-IR)
Spectroscopy
The tool used to record IR spectrum was the
Shimadzu-IR Prestige 21 Spectrometer with
scanning region 400-4000 cm
-1
at 16 cm
-1
resolution.
Samples were mixed with KBr powder and
examined using IR spectrometer.
2.3.3 Scanning Slectron Microscope (SEM)
The morphology of the surface of the Al-CMC-BS
film was discovered by using electron microscope
(Bruker) with a magnification of 500 times under
10.00 kV of voltage
2.3.4 Differential Scanning Calorimetry
(DSC)
To measure Tg, differential scanning calorimetry
(DSC), type METTLER TOLEDO (Switzerland),
model DSC1, temperature range: 25-3000
0
C,
Heating rate:10 K/ min, N
2
gas 50 mL/min.
3 RESULT AND DISCUSSION
3.1 Isolation of Breadfruit Starch
In this study the starch used was breadfruit starch, of
which 600 g of breadfruit produced 60 g of starch.
Synthesis and Characterization of Superabsorbent Polymer based on Carboxymethyl Cellulose, Breadfruit Starch and Aluminum Sulfate
117
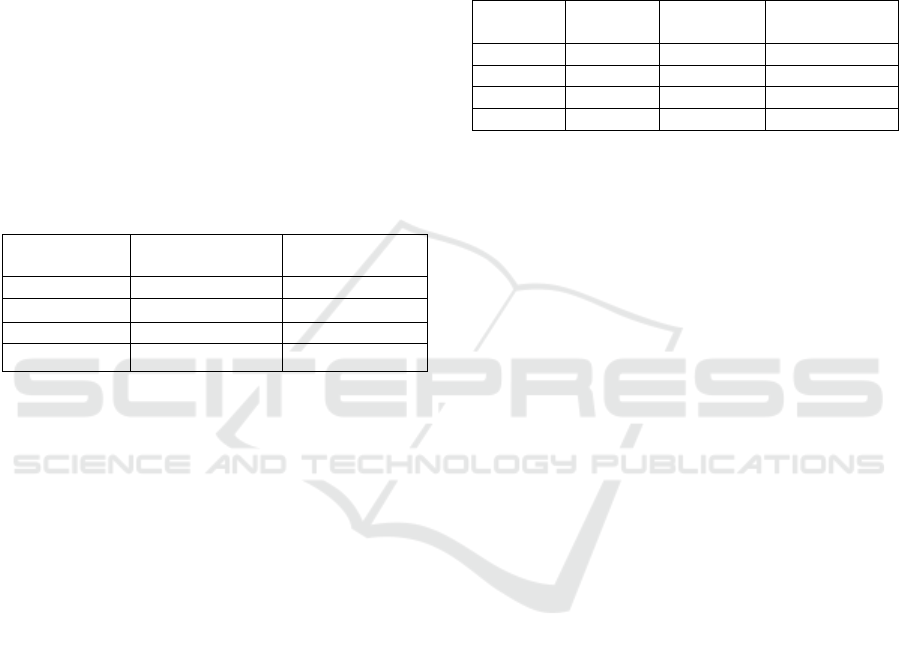
The FT-IR spectrum of breadfruit starch is shown in
Fig.1. The absorption peak at 3387 cm
-1
showed a
stretching group (O-H). The absorption peak of 2924
cm
-1
indicates the presence of a C-H (-CH
2
) group.
Almost the same OH and CH (-CH
2
) absorption
peaks from breadfruit starch were also obtained by
Nurhaeni et.al at wave number 3377.37 cm
-1
at
2927.27 cm
-1
The absorption peak at wave number
1157 cm
-1
was absorption peak of C-O alcohol and
absorption peak at 1018 cm
-1
was the C-O glycosidic
functional group (Nurhaeni et al., 2018). This
spectrum shows that the spectrum in Fig. 1 is a
spectrum of carbohydrate compounds (starch).
3.2 The Cross-Linked Al-CMC
Water absorption capacity from the Cross-Linked
Al-CMC can be seen in Table 1.
Table 1: WAC Values from The Cross-Linked Al-CMC.
Na – CMC
(g)
Al
2
(SO
4
)
3
18 H
2
O
(g)
Al-CMC (%)
2.8
0.0
772.22
2.8
0.2
1911.11
2.8
0.6
1500.01
2.8
0.8
1322.22
The test was carried out by immersing 0.1 g of
Al-CMC in a tea bag which weighed 0.8 g and then
immersed in 20 mL distilled water. Based on table 1,
it can be seen that the WAG value of Na-CMC is the
lowest that is equal to 772.22%.When the the
concentration of the cross-linker is low , it leads to
low degree of cross linking, and it is hard for
network structure to form, so the water absorbency
is low. However, when cross-linker is higher than
2%, there are much more cross-linking points and
the pores become smaller in the network, which
causes the decrease of the water absorbency (Braihi,
2015). The optimum absorption capacity of Al-CMC
is in the ratio of 2.8: 0.2 or (98: 2) % of 1911.11 %
/0.1 g Al-CMC (191.11 g water / g Al-CMC-BS)
3.3 The Cross-Linked Al-CMC-BS
The WAC values from the cross-linked Al-CMC-BS
on various compositions can be seen in table 2. The
cross-linked Al-CMC-BS is made by mixing the
cross-linked Al-CMC that has the optimum WAC
price, which is the ratio of Na- CMC against
aluminum sulfate octadecahydrate 2.8: 0.2 (see table
1) with breadfruit starch. From table 2 it can be seen
that the addition of breadfruit starch gives a very
significant increase in WAC and the optimum WAC
value is in the composition 2.8: 0.2: 0.5. that is equal
to 244.444% / g Al-CMC-BS. Breadfruit starch
serves to increase the number of hydrophilic groups
and form a network structure that is larger than the
Al-CMC network structure so that the absorption of
water increases (Braihi, 2015).
Table 2: Values of WAC from the Cross-Linked Al-CMC-
BS
Na – CMC
(g)
Al
2
(SO
4
)
3
18 H
2
O (g)
BS (g)
Al-CMC-BS (%)
2.8
0.2
0.0
772.22
2.8
0.2
0.5
2444.44
2.8
0.2
1.0
2360.00
2.8
0.2
1.5
2356.66
The broad peak at 3300-3450 cm
-1
is presented
by O-H stretching and peak that appears at 2900-
3000 cm
- 1
. is saturated aliphatic C-H group of CMC
The peak around 1060 cm
-1
is due to C-O-C
stretching and peak at 1604.77 cm-1 is assigned to
stretching vibration of the carbonyl group from
COO-.
The small peaks detected at 894.97 cm-1 were
confirmed to β 1-4 glycoside bonds. The existence
of the carbonyl group and its salt that assigned to
carboxymethyl group was observed at 1600-1640
cm
-1
and 1400-1450 cm
-1
(Siregar et al., 2019).This
proves that this spectrum is a spectrum of Na-CMC.
At Al-CMC-BS there is a change in the ether (CH2
O CH2) group at wave number 1327.03 cm-1 after
addition of starch. This is due to the presence of a
strong absorption band on carbonil groups of
1635.64 cm-1 where the absorption band has a
strong influence on the strain of strong carbonil
bonds so that a large dipole moment is needed.
Wave number 1118.71 cm-1 with the C-O-C group
is a weak asymmetric strecthing bond at wave
number 848.64 cm-1. At wave number 2924.09 cm-
1 with the C-H group with the stretching group CH2,
the wave number 1373.32 cm-1 is a wave number
which indicates a bending of the -OH vibration.
Figure 3 is a DSC thermogram from samples.
Investigation with DSC aims to determine whether
Al-CMC-BS is a miscible polymer mixture. The
presence of one Tg price indicates that the polymer
mixture is miscible and if two Tg prices indicate the
polymer mixture is immiscible. Curve shows that
there is one glass transition value (Tg) on Al-CMC-
BS that is at 95.85˚C, which is between Tg from BS
at 118.72˚C and Tg from CMC at 94.23˚C. This
shows that the mixture of CMC and BS is miscible
or Al-CMC-BS is a miscible mixture (Braihi, 2015).
ICOCSTI 2019 - International Conference on Chemical Science and Technology Innovation
118
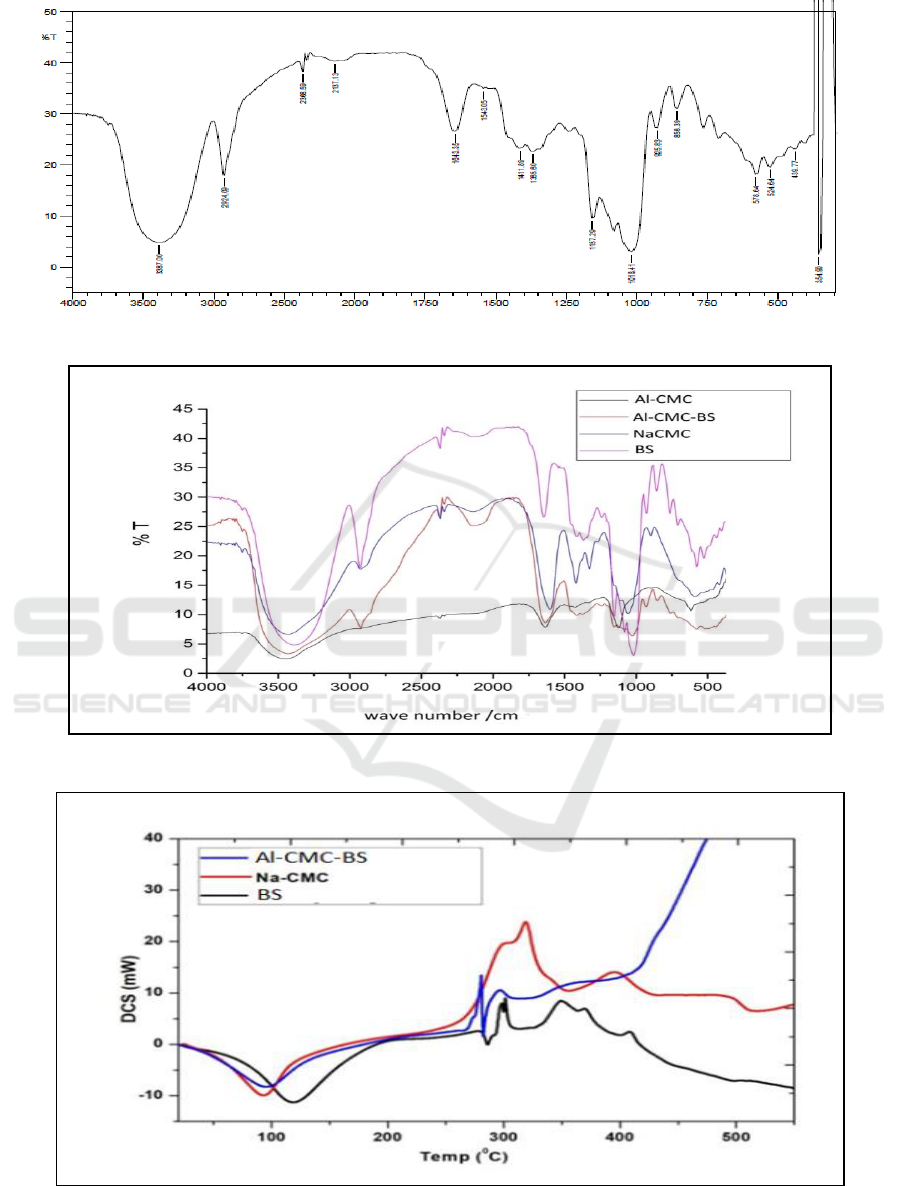
Figure 1: FTIR Spectrum of Breadfruit Fruit Starch.
Figure 2: Increasing FT-IR Spectrum BS, Na-CMC, Al-CMC and Al-CMC-BS
Figure 3: Termogram DSC of Al-CMC-BS, Na-CMC and BS.
Synthesis and Characterization of Superabsorbent Polymer based on Carboxymethyl Cellulose, Breadfruit Starch and Aluminum Sulfate
119

Figure 4.is a SEM photo of Na-CMC and Al-CMC-
BS with optimum WAG. SEM photos showed that
Al-CMC-BS had a coarser and more porous surface
observed compared to surface SEM photos from Na-
CMC. This shows the formation of polymeric
networks that are intertwined and interact with each
other. Larger pore presence in the AL-CMC-BS will
facilitate more water absorption and retention
(Pourjavadi et al., 2007)(Ma et al., 2015)
(a) (b)
Figure 4: (a) Photograph SEM (a) Na-CMC and (b) Al-
CMC-
4 CONCLUSIONS
A super absorbent polymer material from CMC,
Aluminum sulfate octadecahydrate and BS called
Al-CMC-BS superabsorbent polymer has been
successfully synthesized. The existence of readfruit
starch is very influential in increasing the absorption
of it to water which reaches 244.4 g of water / g Al-
CMC-BS. Al-CMC-BS super absorbent polymer is a
miscible porous blend
ACKNOWLEDGEMENTS
Author would like to thank to Rector of University
of Sumatera Urara for the funding from the project
of PD-TALENTA 2019
REFERENCES
Abdel-raouf, M.E., 2019. Guar gum based hydrogels for
sustained water release applications in agriculture, a
review. Curr. Res. Biopolym. 2, 1–15.
Braihi, A.J., 2015. Proposed Cross-Linking Model for
Carboxymethyl Cellulose /Starch Superabsorbent
Polymer Blend. Int. J. Mater. Sci. Appl. 3, 363.
Chandra Sutradhar, S., Mizanur, M., Khan, R., 2015.
Synthesis of superabsorbent polymer from
carboxymethylcellulose/acrylic acid blend using
gamma radiation and its application in agriculture
Electrolyte preperation View project. Artic. J. Phys.
Sci. 26, 23–39.
Chatterjee, P.K., 2002. Absorbent Technology, Textile
Science and Technology.
Klinpituksa, P., Kosaiyakanon, P., 2017. Superabsorbent
Polymer Based on Sodium Carboxymethyl Cellulose
Grafted Polyacrylic Acid by Inverse Suspension
Polymerization. Int. J. Polym. Sci.
Ma, G., Yang, Q., Ran, F., Dong, Z., Lei, Z., 2015. High
performance and low cost composite superabsorbent
based on polyaspartic acid and palygorskite clay.
Appl. Clay Sci.
Masita, S., 2017. Characteristics Of Physico Chemical
Properties Of Breadfruit Flour (Artocarpus altilis)
With Toddo’puli Varieties. J. Pendidik. Teknol.
Pertanian, 3, 234–241.
Mechtcherine, V., 2016. Use of superabsorbent polymers
(SAP) as concrete additive. RILEM Tech. Lett.
Nnadi, F., Brave, C., 2011. Environmentally friendly
superabsorbent polymers for water conservation in
agricultural lands. J. Soil Sci. Environ. Manag.
Nurhaeni, Pratiwi, D., Prismawiryanti, 2018. Modified
Breadfruit (Artocarpus altilis) Starch Using Acetic
Acid Anhydride and Its Application for Noodles
Production. Kovalen J. Ris. Kim. 4, 33–40.
Pourjavadi, A., Amini-Fazl, M.S., Ayyari, M., 2007.
Optimization of synthetic conditions CMC-g-poly
(acrylic acid)/Celite composite superabsorbent by
Taguchi method and determination of its absorbency
under load. Express Polym. Lett. 1, 488–494.
Raju, K.M., Raju, M.P., Mohan, Y.M., 2003. Synthesis of
superabsorbent copolymers as water manageable
materials. Polym. Int.
Sadeghi, M., Soleimani, F., 2013. Synthesis and
Characterization Superabsorbent Hydrogelsfor Oral
Drug Delivery Systems. Int. J. Chem. Eng. Appl.
Salavati, S., Valadabadi, S.A., Parvizi, K. H., Sayfzadeh,
S., Hadidi Masouleh, E., 2018. The effect of super-
absorbent polymer and sowing depth on growth and
yield indices of potato (Solanum tuberosum L.) in
Hamedan Province, Iran. Appl. Ecol. Environ. Res.
Siregar, F.K., Muis, D. Y. N. Y., Kaban, D. Y., 2019.
Preparation And Characterization Of Antibacterial
Film Based On Carboxymethylcellulose From Gebang
Leaf ( Coryphautan ), POLYVINYL ALCOHOL
ANDCITRIC ACID 12, 554–564.
Tao, J., Zhang, W., Liang, L., Lei, Z., 2018. Effects of
eco-friendly carbohydrate-based superabsorbent
polymers on seed germination and seedling growth of
maize. R. Soc. Open Sci.
Weerawarna, S.A., 2009. ( 2 ) Patent Application
Publication ( 10 ) Pub . No .: US 2009 / 0014391 A1 1
ICOCSTI 2019 - International Conference on Chemical Science and Technology Innovation
120
