
Chemical Compounds from Fungus Syncephalastrum racemosum
Isolated as Endophytic from Ageratum conyzoides
Elfita
1*
, Muharni
1
, Mardiyanto
2
, Fitrya
2
and Rismawati Simangunsong
2
1
Department of Chemistry, Faculty of Mathematics and Natural Sciences, University of Sriwijaya, Inderalaya,
Kabupaten Ogan Ilir 30662, Indonesia
2
Department of Pharmacy, Faculty of Mathematics and Natural Sciences, University of Sriwijaya, Inderalaya,
Kabupaten Ogan Ilir 30662, Indonesia
Keywords: Ageratum conyzoides, Endophytic Fungus, Syncephalastrum racemosum.
Abstract: Ageratum conyzoides known as bandotan is a plant widely grown in Indonesia. This plant is used for the
treatment of various diseases such as antibacterial, anti-diabetic, anti-inflammatory, antioxidant, and
analgesic. The active compounds contained in this plant include alkaloids, flavonoids, tannins, glycosides,
minerals and other compounds. Plants that have ethno medicine history are promising candidates to obtain
bioactive compounds from their endophytic fungi. In the present study, chemical compounds were isolated
from endophytic fungus Syncephalastrum racemosum from the stem of Ageratum conyzoides with the
chromatography method. The structures of the compounds were determined by spectroscopy analysis. The
compounds are aromatic group.
1 INTRODUCTION
Ageratum conyzoides is known as bandotan. In some
countries, bandotan is considered a weed plant and
its growth is very fast. This plant comes from
tropical America, especially Brazil. Most of the A.
conyzoides plants are found in Mexico, Central
America, the Caribbean Islands, and Florida. But
now bandotan is also found in several sub-tropical
and tropical countries, including in Indonesia.
Bandotan plants are now widespread in various parts
of Indonesia. Ageratum conyzoides often grow in
yards, roadside, fields, dry rice fields, river banks,
and areas with a lot of shrubs. This plant has a long
history in its use for traditional medicine in several
countries. This plant has medicinal bioactive
properties. Therefore bandotan plants can be
classified as herbal plants (Soerjani et al., 1987;
Darma, 1987; Singh et al., 2013; Odeleye et al.,
2014; Janarthanan et al., 2016)
In general A. conyzoides contains a variety of
bioactive compounds including flavonoids,
alkaloids, coumarins, essential oils, tannins,
chromene, benzofuran and terpenoids. All parts of
this plant have the ability to be anti-inflammatory
and anti-allergic. In addition, antidiareal,
nematoside, anticoagulant, smooth muscle relaxant,
hemostatic, analgesic, antifungal, antibacterial, and
hypothermic factors are also reported (Kamboj and
Saluja, 2008; Ndip et al., 2009; Awad et al., 2013;
Bahtiar et al., 2017)
In Bogor, A. conyzoides is widely known as a
wound medicine. According to Heyne, these plant
leaves are squeezed, mixed with lime, applied to
fresh wounds. Decoction of leaves is also used to
treat chest pain, while extracting the leaves for eye
drops. Mashed roots are applied to the body to treat
fever, the extract can be drunk. bandotan also to treat
stomach ache and to cure broken bones (Heyne,
1987; Darma, 1987).
Endophytic fungi are microorganisms that live to
form colonies in plant tissues without endangering
their host plants. Each high-level plant contains
several endophytic microbes which produce
secondary metabolites as a result of coevolution or
genetic transfer (genetic recombination) from the
host plant to endophytic fungi. The ability of
endophytic fungi to produce phytochemical
compounds that are also produced by their host
plants may be related to the presence of genetic
recombination of endophytic fungi with hosts during
the time of their evolution (Elfita et al, 2013; Sandhu
et al., 2014; Golinska et al., 2015).
136
Elfita, ., Muharni, ., Mardiyanto, ., Fitrya, . and Simangunsong, R.
Chemical Compounds from Fungus Syncephalastrum racemosum Isolated as Endophytic from Ageratum conyzoides.
DOI: 10.5220/0008858001360140
In Proceedings of the 1st International Conference on Chemical Science and Technology Innovation (ICOCSTI 2019), pages 136-140
ISBN: 978-989-758-415-2
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

Endophytic fungi are a source of genetic
diversity with various possible new species that have
not been described. Therefore, the need for natural
products for new antibiotics, chemotherapy and
agrochemicals that have high activity, low toxicity,
but do not disturb environmental ecology can be
expected to be obtained from this endophytic fungus
(Rajamanikyam et al., 20017; Kaur et al., 2018;
Santoyo et al., 2016; Elfita et al., 2015).
Previous studies have reported six endophytic
fungi isolated from the leaves and stems of
Ageratum conyzoides (Elfita et al., 2019). Screening
the antibacterial activity of ethyl acetate extract from
liquid culture showed that the Syncephalastrum
racemosum fungus had the highest activity. In this
paper, the chemical compounds contained in the
antibacterial active extract of the S. racemosum
fungus are reported.
2 MATERIALS AND METHODS
2.1 Chemicals
The materials used in this study include endophytic
fungi, Syncephalastrum racemosum which have
been previously isolated, Potato Dextrose Agar
(PDA), Potato Dextrose Broth (PDB), alcohol 70%,
KLT kiessel gel 60 F254 20 x 20 cm, silica gel G 60
70-230 mesh. Organic solvents such as n-hexane,
ethyl acetate and methanol.
2.2 Source of Endophytic Fungi
Syncephalastrum racemosum of Ageratum
conyzoides from stock fungus (Elfita et al., 2019).
The fungal was identified molecularly in the
biological research center-LIPI Cibinong. The
Ageratum conyzoides was collected in Juli 2018
from Indralaya, Ogan Ilir, South Sumatra.
2.3 Cultivation and Extraction of
Endophytic Fungi S. racemosum
The suspension of endophytic fungus (which was
previously isolated) was prepared by taking 6 ose of
endophytic fungi, then inoculated into 200 mL of
PDB (in 5x 1 liter bottles). They were incubated at
room temperature and static conditions for 6 weeks.
Furthermore, endophytic fungal cultures are
harvested by separating biomass. the liquid culture
was extracted using ethyl acetate in a separating
funnel. then the extract was evaporated using a
rotary evaporator to obtain concentrated ethyl
acetate extract (Marcellano et al., 2017; Elfita et al.,
2012).
2.4 Isolation of Secondary Metabolites
and Identification of Structures
The ethyl acetate concentrate extract of endophytic
fungi was separated by chromatography column.
The sample was prepared by preadsorption and put
into the column over silica gel 70-230 mesh.
separation was carried out using using eluents with
gradient system (n-hexane-EtOAc-methanol). The
eluates was collected with a vial (10 mL) and
analyzed by TLC under UV lamp. The eluate with
the same stain pattern was combined into one
fraction. The major and fluorinated stain fractions
were then separated and purified. Sub-fractions were
separated again using column chromatography over
silica gel (70-230 mesh) with gradient eluents.
Eluate is collected in a vial (5 mL volume) and
analyzed by TLC. Eluat with the same stain pattern
were combined into one fraction. The subfraction re-
purified column chromatography to obtain the pure
compounds. The compounds were analyzed for
their chemical structure using spectroscopic
methods.
3 RESULT AND DISCUSISON
3.1 Isolation of Chemical Compounds
The filtrate was evaporated by rotary evaporator to
give a EtOAc extract (4.1 g). The extract (4.1 g) was
separated over a silica gel column (70.230 mesh, 40
g) with gradient solvent system of n-
hexane/EtOAc/MeOH as the eluent to give five
fractions (A1–A5). Fraction A1 (2.34 g) was
subjected to column chromatography (CC) eluted
with n-hexane-EtOAc (10:0→0:10) as to give four
sub-fractions (A11–A14). Subfraction A12 (1.1 g)
was subjected to CC over a silica gel (70-230 mesh,
20 g) eluted with with n-hexane-EtOAc (9:1) to
give compound 1 (479 mg). Fraction A3 (709 mg)
was separated to CC eluted with n-hexane-EtOAc
(7:3→0:10) as to give five sub-fractions (A31–A35).
Subfraction A31 (91 mg) was subjected to CC over a
silica gel (70-230 mesh, 10 g) eluted with with n-
hexane-EtOAc (5:5) to give compound 2 (21 mg).
Subfraction A35 (56 mg) was subjected to CC eluted
with n-hexane-EtOAc (4:6) to give Compound 3 (11
mg, not identified).
Chemical Compounds from Fungus Syncephalastrum racemosum Isolated as Endophytic from Ageratum conyzoides
137
3.2 Identification of Chemical
Compounds
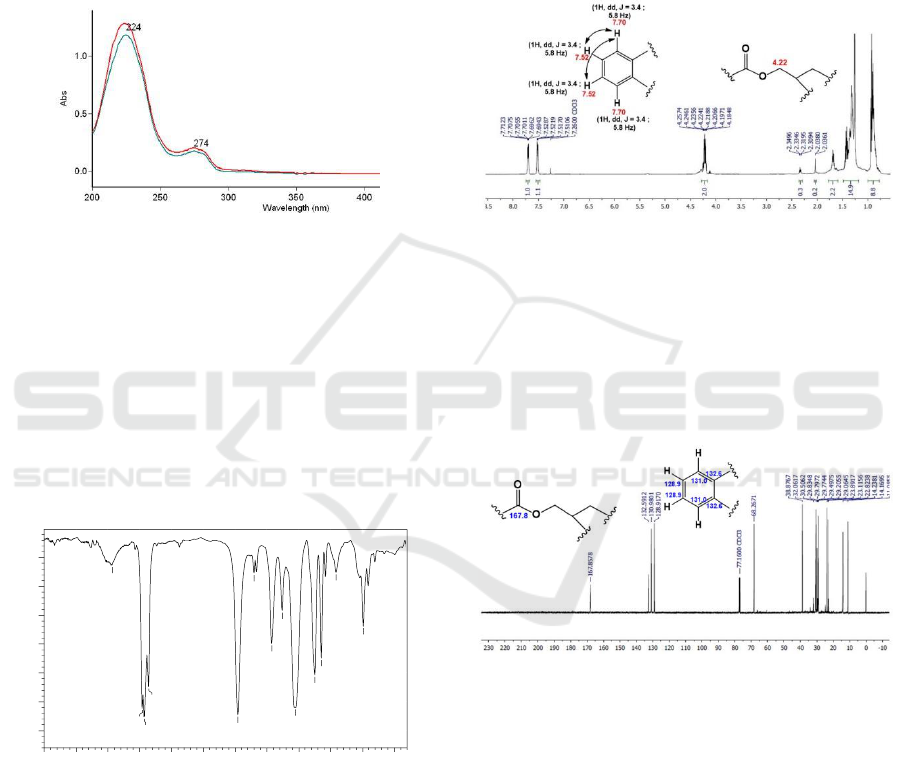
Compound 1 The UV spectrum of compound 1 in
methanol solvents (Figure 1) showed absorption at
λ
max
224 and 274 nm. Addition of NaOH does not
cause a batochromic shift. This indicates the absence
of a free hydroxyl group on the aromatic ring
(Muharni et al., 2014).
Figure 1: The UV spectrum of compound 1.
The FTIR spectrum (Figure 2) showed the
presence of characteristic absorption bands at υ
3344.3 cm
-1
which is absorption for OH, while the
absorption of 2854.7-2958.8 cm
-1
is a typical for
aliphatic C-H. In addition there is absorption in the
area of 1728.22 cm
-1
which is absorption for C = O
bonds. The presence of aromatic C = C is
characterized by absorption at 1600.9 and 1462.04
cm
-1
and typical absorption of C-O ester in the area
of 1274.9 cm
-1
.
Figure 2: The FTIR spectrum of compound 1.
Figures 3 showed the presence of 6 proton
signals. The signal at
H
0,89 ppm (12H, m) for 4
methyl groups. Furthermore, the signal that
accumulates in the area around
H
1,30-140 ppm (
18H, m) showed the presence of aliphatic CH
2
groups. In the
1
H-NMR spectrum also shows that the
signal in the area of 1.68 ppm (2H, m) is a signal for
two CH groups coupled by protons through three
bonds.
The signal at
H
4,22 ppm (2H, m) showed the
presence of O-CH
2
group (2 CH
2
groups).
Furthermore, the signal in the area of
H
7,52 and
H
7,70 ppm (2H, dd J = 3.4 and 5.8 Hz) respectively
showed the presence of four aromatic protons
(coupled meta and ortho). Each signal represented
two protons. the compound 1 as an aromatic ring in
the form of symmetrical disubstitution (Habib and
Karim, 2009).
Figure 3: The
1
H-NMR spectrum of compound 1 (500
MHz
1
H- in CDCL
3
.
The
13
C-NMR spectrum (Figure 4) showed the
presence of 12 signals consisting of 8 C sp3 signals
that appear below 100 ppm (
C
11.1; 14.2;
23.1;
23.9;
29.1;
30.5;
38.9; and 68.3 ppm ) and 4 other
signals that appear above 100 ppm (
C
128.9;
131.0;
132.6; and
167.8 ppm). are signals for C sp
2
.
Figure 4: The
13
C-NMR spectrum of compound 1 (125
MHz
13
C- in CDCL
3
).
The
1
H-NMR spectrum of compound 2 (Figure 5)
showed a group of protons similar to compound 1. It
appears that compound 2 also has signals in the
regions
H
7,53 dan
H
7,71 ppm (2H, and J = 3.3
and 5.7Hz) respectively showed the presence of four
aromatic protons (coupled meta and ortho). Each
signal represented two protons. The compound 2 as
an aromatic ring in the form of symmetrical
disubstitution. The next similar signal, at
H
4,22
ppm (2H, m) for O-CH
2
group (2 CH
2
groups) and at
H
0.5-2.00 ppm is a long chain of aliphatic protons.
The difference is the appearance of a proton signal at
5007501000125015001750200025003000350040004500
1/cm
-0
15
30
45
60
75
90
%T
3433.29
2958.80
2926.01
2854.65
1728.22
1600.92
1462.04
1381.03
1274.95
1122.57
1072.42
958.62
742.59
Swl2
ICOCSTI 2019 - International Conference on Chemical Science and Technology Innovation
138

H
3.66 ppm which is a methoxyl proton (Habib and
Karim, 2009; Muharni et al., 2014).
Figure 5: The
1
H-NMR spectrum of compound 2 (500
MHz
1
H- in CDCL
3
).
4 CONCLUSIONS
Chemical compounds isolated from the ethyl acetate
extract of liquid cultured of endophytic fungi
Syncephalastrum racemosum were identified as
phthalate derivatives.
ACKNOWLEDGMENTS
The authors are thankful to ministry Ristekdikti
Republic of Indonesia, which provides funding with
the research grant Penelitian Dasar Unggulan
Perguruan Tinggi (PDUPT) 2019.
REFERENCES
Awad, N. E., Kassem, H. A., Elkhayat, Z. A., El-feky, A.
M., Matloub, A. A., 2013. Chemical composition and
anti-inflammatory evaluation of Ageratum conyzoides
L. leaves. J. Appl. Sci. Res. 9 (3), 2126-2134.
Bahtiar, A., Nurazizah, M., Roselina, T., Tambunan, A. P.,
Arsianti, A., 2017. Ethanolic extracts of babandotan
leaves (Ageratum conyzoides L.) prevents
inflammation and proteoglycan degradation by
inhibiting TNF-a and MMP-9 on osteoarthritis rats
induced by monosodium iodoacetate. APJTM. 10 (3),
270-277.
Darma, A. P., 1987. Indonesian Medicinal Plants
[Tumbuhan Obat Indonesia]. Balai Pustaka, Jakarta,
hal. 28 – 29.
Elfita, E., Munawar, M., Muharni, M., Ivantri, I., 2015.
Chemical constituens from an endophytic fungus
Aspergillus sp-2 isolated from sambiloto
(Andrographis paniculata Nees). Microbiol. Indones,
9 (2), 82-88.
Elfita, E., Muharni, M., Munawar, M., and Rizki, R.,
2012. Isolation of antioxidant compound from
endophytic fungi Acremonium sp from the twigs of
Kandis Gajah (Garcinia griffithii T, Anders). Makara
of Science Series, 16 (1), 46-50.
Elfita, E., Munawar, M., Muharni, Suprayetno, S., 2013.
New pyran of an endophytic fungus Fusarium sp.
isolated from the leaves of brotowali (Tinaspora
crispa). Indo J Chem., 13, 209-215.
Elfita, E., Muharni, M., Mardiyanto, M., Fitrya, F.,
Nurmawati, E., and Simangungsong, R., 2019.
Antibacterial activity of Ageratum conyzoides and
their endophytic fungi extracts. Microbiology and
Biotechnology Letter. (In Press).
Golinska, P., Wypij, M., Agarkar, G., Rathod, D., Dahm,
H., & Rai, M., 2015. Endophytic actinobacteria of
medicinal plants: Diversity and bioactivity. Antonie
Van Leeuwenhoek International Journal of General,
108 (2), 267–289.
Heyne, K., 1987. Tumbuhan berguna Indonesia, jilid. 3:.
Terj. Yayasan Sarana Wana Jaya, Jakarta, p. 1825-
1826.
Habib, M. R. and Karim, M. R., 2009. Antimicrobial and
Cytotoxic Activity of Di-(2-ethylhexyl) Phthalate and
Anhydrosophoradiol-3-acetate Isolated from
Calotropis gigantea (Linn.) Flower. Mycobiology
37(1), 31-36.
Janarthanan, L., Karthikeyan, V., Jaykar, B., Balakrishnan,
B. R., Senthilkumar, K. L., and Anandharaj, G., 2016.
Pharmacognostic studies on the whole plants of
Ageratum conyzoides Linn. (Asteraceae). EJPMR. 3
(5), 618-626.
Kamboj, A., Saluja, A. K., 2008. Ageratum conyzoides L.:
A review on its phytochemical and pharmacological
profile. IJGP. 2 (2), 59-68.
Kaur, J., Kaur, R., & Kaur, A., 2018. Evaluation of
antidiabetic and antioxidant potential of endophytic
fungi isolated from medicinal plants. International
Journal of Green Pharmacy, 12 (1), 6-12.
Marcellano, J. P., Collanto, A. A., and Fuentes, R. G.,
2017. Antibacterial activity of endophytic fungi
isolated from the bark of Cinnamomum mercadoi.
Pharmacogn J., 9(3), 405-409.
Muharni, M., Fitrya, F., Ruliza, M. O., Susanti, D. A., and
Elfita, E., 2014. Di-(2-ethylhexyl) phthalate and
pyranone derivated from endophytic fungi Penicillium
sp the leave of Kunyit Putih (Curcuma zedoaria).
Indonesian Journal of Chemistry, 14 (3), 290 – 296.
Ndip, R. N., Ajonglefac, A. N., Wirna, T., Luma, H. N.,
Wirmum, C., Efange, M. N., 2009. In-vitro
antimicrobial activity of Ageratum conyzoides (Linn)
on clinical isolates of Helicobacter pylori. Afr. J.
Pharm. Pharmacol. 3 (11), 585-592.
Odeleye, O. P., Oluyege, J. O., Aregbesola, O. A.,
Odeleye, P. O., 2014. Evaluation of preliminary
phytochemical and antibacterial activity of Ageratum
conyzoides (L) on some clinical bacterial isolates.
IJES. 3 (6): 01-05.
Rajamanikyam, M., Vadlapudi, V., Amanchy, R.,
Upadhyayula, S. M., 2017. Endophytic fungi as novel
Chemical Compounds from Fungus Syncephalastrum racemosum Isolated as Endophytic from Ageratum conyzoides
139

resources of natural therapeutics. Braz. Arch. Biol.
Technol., 60, 1-26.
Santoyo, G., Hagelsieb, M. G., Mosqueda, D. C. O. M., &
Glick, B. R., 2016. Plant growth-promoting bacterial
endophytes. Microbiological Research, 183, 92–99.
Sandhu, S. S., Kumar, S., Aharwal, R. P., 2014. Isolation
and identification of endophytic fungi from ricinus
communis linn. and their antibacterial activity. IJRPC,
4(3), 611-618.
Singh, S. B., Devi W. R., Marina, A., Devi, W. I.,
Swapana, N., Singh, CB., 2013. Ethnobotany,
phytochemistry and pharmacology of Ageratum
conyzoides Linn (Asteraceae). J. Med. Plants Res. 7
(8): 371-385.
Soerjani, M., Kostermans, A. J. G. H., Tjitrosoepomo, G.,
1987. Weeds of rice in Indonesia. Balai Pustaka,
Jakarta, p. 60-61
ICOCSTI 2019 - International Conference on Chemical Science and Technology Innovation
140
