
Cytotoxicity of Heterophyllene A, the Derivative of Arylbenzofuran
from Stem Bark of Artocarpus calophylla
Rumzil Laili
1*
, Nanik Siti Aminah
2
, Tin Myo Thant
2
, Yoshiaki Takaya
3
, Ainun Sa’adah
1
, Alfinda
Novi Kristanti
2*
1
Student of Magister Chemistry Program, Science and Technology Faculty, Airlangga University, Jl. Ir Soekarno,
Surabaya, 60115
2
Chemistry, Science and Technology Faculty, Airlangga University, Jl. Ir Soekarno, Surabaya, 60115
3
Faculty of Pharmacy, Meijo University, 468-8503, Tempaku Nagoya, Japan
ainun.saadah2@gmail.com, alfinda-n-k@fst.unair.ac.id
Keywords: Artocarpus calophylla, Arylbenzofuran, Cytotoxicity
Abstract: Exploration of secondary metabolites was the focus of this research, especially of Artocarpus calophylla
species to look for a potential cytotoxic agent. An arylbenzofuran derivative compound, namely
heterophyllene A was isolated from the stem bark of Artocarpus calophylla. Structure determination of this
compound has been elucidated using UV-Vis spectroscopy, 1D, and 2D NMR analysis. This compound has
a lower IC
50
than ethyl acetate extract. The IC
50
of this compound (57,54 µg/mL) to HeLa and (25,80
µg/mL) to T47D cells, ethyl acetate extract (>100 µg/mL) to HeLa and (84,16 µg/mL) to T47D cells.
1 INTRODUCTION
Moraceae is a family of plants that is a source of a
bioactive compound in large quantities. The main
genus in Moraceae is Artocarpus which consists of
more than 60 species. Artocarpus plants spread from
Southest Asia, South Asia, Nothern Australia and
Central America (Kochummen 1987; Verheij and
Coronel, 1992). Some Artocarpus species commonly
found in Indonesia include jackfruit (A.
heterophyllus Lamk), cempedak (A. champeden),
breadfruit (A. altilis [Park] Fosberg) (Ilyas, 2013)
and others which are endemic in Myanmar such as
A. lakoocha and A. calophylla KURZ (Takahashi et
al., 2004).
There are some secondary metabolites which are
proven capable to be produced by this genus, for
instance, terpenoid, steroid, and phenolic compound
(Barik et al., 1997; Wang et al., 2007; Chen et al.,
2010; Nguyen et al., 2012). A number of
pharmacologically active constituents have been
isolated from Artocarpus species, with this having a
variety of activities including antibacterial (Khan et
al., 2003), antiplatelet (Weng et al., 2006),
antifungal (Jayasinghe et al., 2004), antimalarial
(Widyawruyanti et al., 2007; Boonlaksiri et al.,
2000) and cytotoxic (Ko et al., 2005; Hakim et al.,
2002; Syah et al., 2006).
In this study, it was reported that heterophyllene
A is an arylbenzofuran derivative compound isolated
from ethyl acetate extract of the stem bark of A.
calophylla. A. calophylla is one of the species in the
genus Artocarpus that has not been widely studied
both from the study of phytochemicals and its
biological activity. The chemical structure of the
compound was determined by UV, 1D, and 2D
NMR. Cytotoxic activity of the compound and ethyl
acetate extract to HeLa and T47D cells is also
described.
2 EXPERIMENTALS
2.1 General
NMR spectra were recorded on JEOL 600 ECA
spectrometer using CDCl
3
at 600 (
1
H) and 125 (
13
C)
MHz. The UV spectrum was recorded using UV-
1800 Shimadzu spectrophotometer. Vacuum Liquid
Chromatography (VLC) and Gravity Column
Chromatography (GCC) were carried out using Si
gel 60 GF254. Meanwhile, Si gel PF254 was used in
156
Laili, R., Siti Aminah, N., Myo Thant, T., Takaya, Y., Sa’adah, A. and Novi Kristanti, A.
Cytotoxicity of Heterophyllene A, the Derivative of Arylbenzofuran from Stem Bark of Artocarpus calophylla.
DOI: 10.5220/0008863201560159
In Proceedings of the 1st International Conference on Chemical Science and Technology Innovation (ICOCSTI 2019), pages 156-159
ISBN: 978-989-758-415-2
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

TLC analysis and pre-coated silica gel plates
(Merck, Darmstadt, Germany, Kieselgel 60 GF254
0,25 mm thickness).
2.2 Plant Material
The sample collection of the stem bark of A.
Calophylla was carried out in April 2018. The
location where the samples were collected was in
Myanmar particularly in Inaw Village, Myitkyina
City. Afterward, before being dried up, the stem
barks were cleaned out. The drying was done in the
shade. After the cleaning and the drying process, it
was then cut into small pieces before finally being
ground into powder.
2.3 Extraction and Isolation
The stem barks of A. calophylla which were already
in the form of powder were macerated with
methanol for three whole days at indoor
temperature. Afterward, the filtration step was done
to obtain the necessary solvent. In order to acquire
the methanol extract, the solvent was then
evaporated using Rotary Vacuum Evaporator. The
crude methanol extract was partitioned with n-
hexane and ethyl acetate. A vacuum liquid
chromatography was utilized to separate as much as
15g of Ethyl acetate extract. The separation process
was done on a silica gel using a mixture of ethyl
acetate and eluent n-hexane by intensifying the
polarity of the gradient. The compound purification
process was done using gravity column
chromatography on silica gel by increasing the
eluent polarity. The compound purity test was
carried out using TLC analysis with at least three
different system and anisaldehyde reagents.
2.3.1 Heterophyllene A
Brown solids; UV (MeOH) λ
max
219, 340, and 358
nm.
1
H-NMR (CDCl
3
, 600 MHz) δ
H
1.46 (3H (2x),
s, Methyl-10,11), 5.64 (1H, d, J=9,9 Hz, H-6), 6.64
(1H, d, J=9.9 Hz, H-5), 6.77 (1H, dd, J=8.3;2.0, H-
5’), 6.81 (1H, s, H-3), 6.85 (1H, s, H-3’), 6.89 (1H,
s, H-9), 6.97 (1H, d, J=2.0, H-7’), 7.38 (1H, d, J=8.3
Hz, H-4’), 8.11 (1H, s, OH);
13
C-NMR (CDCl
3
, 125
MHz), δ
C
27.8 (CH
3
(2x), C-10;C-11), 76.3 (C, C-7),
98.1 (CH, C-7’), 101.5 (CH, C-3’), 103.9 (CH, C-3),
105.5 (CH, C-9), 109.6 (C, C-4a), 112.1 (CH, C-5’),
116.1 (CH, C-5), 117.6 (C, C-3a), 121.2 (CH, C-4’),
122.8 (C, C-3a’), 129.6 (CH, C-6), 151.2 (C, C-2),
151.4 (C, C-4), 153.6 (C (2x), C-2’, C-7a’), 154.2
(C, C-8a), 154.7 (C, C-9a), 155.6 (C, C-6’).
2.4 Cytotoxicity Bioassays
The Cytotoxic assay was carried out using MTT
assay method in vitro against HeLa and T47D cells.
Cytotoxic tests were carried out by planting cancer
cell cultures that had been harvested into 96 well
plates. Furthermore, the 96 well plates containing
the cancer cells were treated with 100 µL of the
isolated compound and were incubated for 24 hours.
The test sample varied the concentration of the
solutions by 7 variations starting from the
concentration of 1,5625; 3,125; 6,25; 12,5; 25; 50
and 100 µg/mL and repeated three times (triple).
The positive control used was doxorubicin, a media
control solution consisting of culture media, and cell
control solution consisting of culture and cell media.
The next step was the administration of 100 µL
of MTT reagents to each well after being incubated
for 24 hours. Afterwards, it was incubated again for
3-4 hours in the CO
2
incubator (until formazan
crystals were formed). When formazan crystals have
been formed, the condition of the cell was observed
with an inverted microscope, then as much as 10%
of SDS stopper was added in 0,1 N HCl. Finally, the
96 well plates were wrapped with paper and were re-
incubated overnight.
The next step was using ELISA reader to read
the absorbance value. It was done to find out the
IC
50
value of each test sample. The reading process
of each well’s absorbance was done with a
wavelength of 500-600 nm. By using absorbance
data which were obtained from the measurements,
then it was possible to determine the percentage of
cells inhibited. The determination of IC
50
values was
carried out using linear regressions.
3 RESULTS AND DISCUSSIONS
Heterophyllene A (Figure 1) was obtained as brown
solids (9 mg). The UV spectrum showed maximum
absorbance at 219, 340, and 358 nm, which indicates
the presence of an arylbenzofuran skeleton (Tan,
et.al., 2012). The
1
H-NMR spectrum (Table 1)
shows aromatic signals with the ABX system at δ
H
7.38 (d, J = 8.3 Hz, 1H), 6.97 (d, J = 2.0 Hz, 1H),
6.77 (dd, J = 8.3 Hz and J = 2.0 Hz, 1H). Two
proton signals at δ
H
5.64 (d, J = 9.9 Hz, 1H) and 6.64
(d, J = 9.9 Hz) indicate the presence of
dimethylchromene rings (Boonyaketgoson, et.al.,
2017). The spectrum shows three singlet aromatic
protons at δ
H
6.85 (s, 1H), 6.89 (s, 1H) and 6.81 (s,
1H) and that there is a singlet signal at δ
H
8.11 (s,
1H) which is a hydroxy group. Spectrum
13
C-NMR,
Cytotoxicity of Heterophyllene A, the Derivative of Arylbenzofuran from Stem Bark of Artocarpus calophylla
157
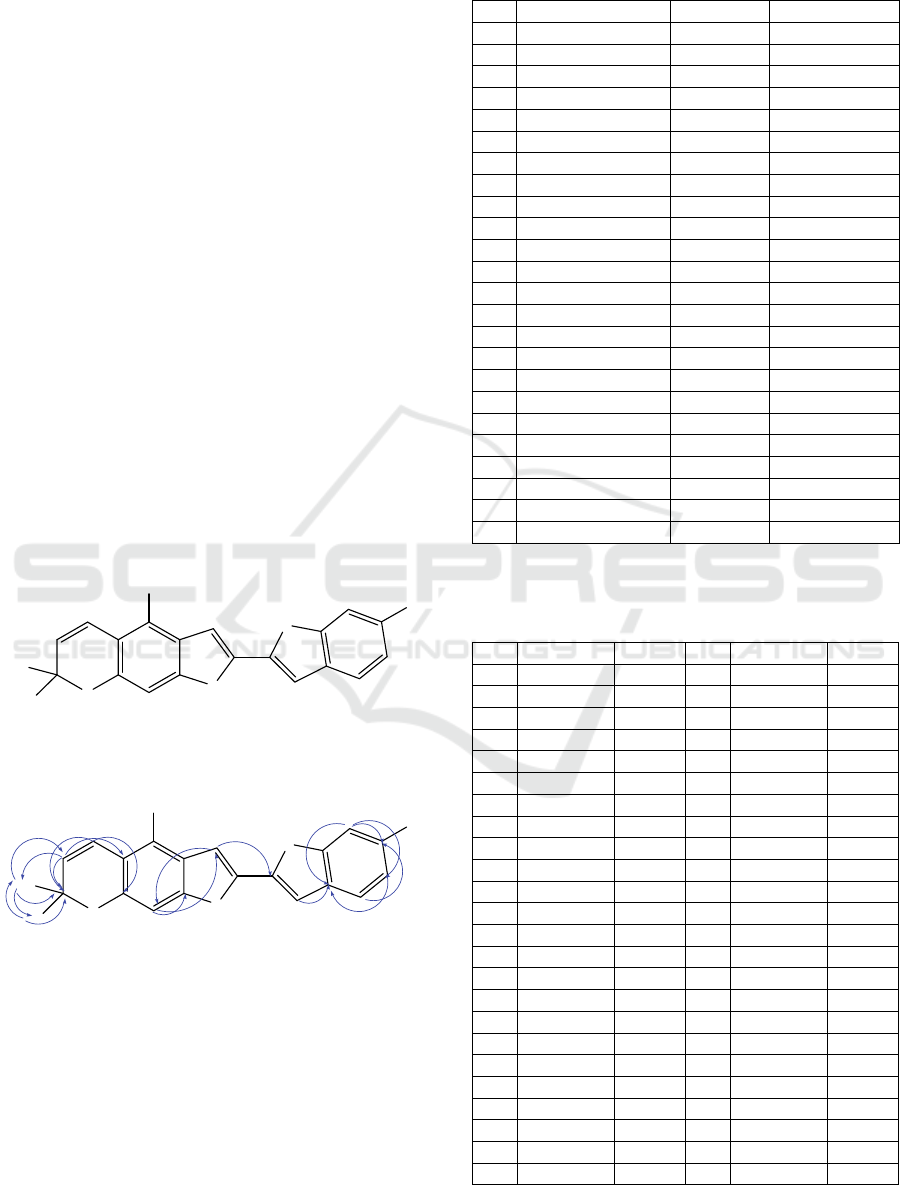
DEPT 90, and DEPT 135 show 21 carbon signals
consisting of eleven quaternary carbon at δ
C
76.3;
109.6; 117.6; 122.8; 151.2; 151.4; 153.6 (2C);
154.2; 154.7; 155.6, two methyl at δ
C
27.8 (2C) and
eight methine at δ
C
98.1; 101.5; 103.9; 105.6; 112.1;
116.1; 121.2; 129.6 (Table 1).
Based on multiplicity and HSQC spectrum, three
aromatic protons which resonate at δ
H
7.38, 6.77 and
6.97 are respectively assigned as H-4’, H-5’, H-7’.
HMBC correlation shows that H-4’ correlates with
C-6’ (Figure 2), H-5’ correlates with C-3’ and C-3a’,
while H-7’ at δ
H
(6.97) correlates with C-3a’, C5’
and C-6’. Singlet signals on aromatic protons at δ
H
6.85 correlates with quaternary carbon at C-3a’, δ
H
6.89 correlates with methine at C-3 and quaternary
carbon at C-9a, δ
H
6.81 correlates with C quaternary
at C-2’ and methine at C-9. This indicates that
singlet aromatic protons are at position C-3’, C-3
and C-9. The hydroxy position was confirmed to be
C-4 and C-6’ by HMBC. Two methyls (C-10 and C-
11) were found by HSQC spectrum to be present in
the compound. Afterward, the authors did additional
evaluation to compare with the published data
(Table 2) (Boonyaketgoson et al., 2017), the
structure of this compound was identified as
Heterophyllene A.
O
O
O
H
3
C
H
3
C
OH
OH
10
11
7
6
5
4a
8a
9
9a
4
3a
3
2
1
2'
1'
3'
7a'
7'
6'
5'
4'
3a'
Figure 1: Structure of Heterophyllene A
O
O
O
H
3
C
H
3
C
OH
OH
10
11
7
6
5
4a
8a
9
9a
4
3a
3
2
1
2'
1'
3'
7a'
7'
6'
5'
4'
3a'
Figure 2: HMBC correlation for Heterophyllene A
Table 1: NMR spectroscopic data of Heterophyllene A in
CDCl
3
No
1
H (m, J in Hz)
13
C (type) HMBC
1 - - -
2 - 151.2 -
3 6.81 (s) 103.9 2’, 9
3a - 117.6 -
4 - 151.4 -
4a - 109.6 -
5 6.64 (d, 9.9) 116.1 7, 8a
6 5.64 (d, 9.9) 129.6 4a, 7, 10
7 - 76.3 -
8 - - -
8a - 154.2 -
9 6.89 (s) 105.5 3, 9a
9a - 154.7 -
10 1.46 (s) 27.8 6, 7, 11
11 1.46 (s) 27.8 6, 7, 10
1’ - - -
2’ - 153.6 -
3’ 6.85 (s) 101.5 3a’
3a’ - 122.8 -
4’ 7.38 (d, 8.3) 121.2 6’
5’ 6.77 (dd, 8.3; 2.0) 112.1 3’, 3a’
6’ - 155.6 -
7’ 6.97 (d, 2.0) 98.1 3a’, 5’, 6’
7a’ - 153.6 -
Table 2: The data of the chemical shift which compares
Heterophyllene A from A. heterophyllus (right) and
Heterophyllene A from A. calophylla (left)
No
1
H (m)
13
C No
1
H (m)
13
C
1 - - 1 - -
2 - 151.2 2 - 154.2
3 6.81 (s) 103.9 3 6.87 (s) 105.6
3a - 117.6 3a - 113.8
4 - 151.4 4 - 151.5
4a - 109.6 4a - 101.2
5 6.64 (d) 116.1 5 6.64 (d) 116.2
6 5.64 (d) 129.6 6 5.64 (d) 129.6
7 - 76.3 7 - 76.3
8 - - 8 - -
8a - 154.2 8a - 154.0
9 6.89 (s) 105.5 9 6.85 (s) 101.6
9a - 154.7 9a - 154.7
10 1.46 (s) 27.8 10 1.40 (s) 27.8
11 1.46 (s) 27.8 11 1.40 (s) 27.8
1’ - - 1’ - -
2’ - 153.6 2’ - 153.6
3’ 6.85 (s) 101.5 3’ 6.97 (s) 98.2
3a’ - 122.8 3a’ - 122.8
4’ 7.38 (d) 121.2 4’ 7.38 (d) 121.1
5’ 6.77 (dd) 112.1 5’ 6.76 (dd) 112.1
6’ - 155.6 6’ - 155.6
7’ 6.97 (d) 98.1 7’ 6.81 103.9
7a’ - 153.6 7a’ - 153.6
ICOCSTI 2019 - International Conference on Chemical Science and Technology Innovation
158

Cytotoxic activity on HeLa and T47D cells
showed that Heterophyllene A compounds were
more toxic than ethyl acetate extracts. The IC
50
of
Heterophyllene A compound was 57.54 µg/mL to
HeLa and was 25.80 µg/mL to T47D cells. The IC
50
of ethyl acetate extract was >100 µg/mL to HeLa
and was 84.16 µg/mL to T47D cells. The IC
50
of
doxorubicin was 2.72 µg/mL to HeLa and was 0.01
µg/mL to T47D cells.
4 CONCLUSIONS
In conclusions, Heterophyllene A, the derivative of
arylbenzofuran compound was isolated from the
stem bark of A. calophylla. The biological activity of
this compound was investigated using citotoxicity
test to HeLa and T47D cells. Although it did not
have strong activity, this compound had better
activity than ethyl acetate extract.
REFERENCES
Barik, B.R., Bhaumik, T., Kundu, A.K., Kundu, A.B.,
1997. Triterpenoids of Artocarpus heterophyllus.
J.Indian Chemical Soc. 74: 163-164.
Boonlaksiri, C., Oonanant, W., Kongsaeree, P., Kittakoop,
P., Tanticharoen, M., Thebtaranonth, Y., 2000. An
antimalarial stilbene from Artocarpus integer.
Tetrahedron Letters. 0040-4039.
Boonyaketgoson, S., Rukachaisirikul, V., Phongpaichit,
S., Trisuwan, K., 2017. Cytotoxic arylbenzofuran and
stilbene derivatives from the twigs of Artocarpus
heterophyllus. Tetrahedron Letters. 0040-4039.
Chen, C.Y., Cheng, M.J., Kuo, S.H., Kuo, S.Y., Lo, W.L.,
2010. Secondary metabolites from the stems of
Artocarpus heterophyllus. Chem.Nat.Comp. 46, 638-
640.
Hakim, E.H., Asnizar., Yurnawilis., Aimi, N., Kitajima,
M., Takayama, H., 2002. Artoindonesianin P, a new
prenylated flavone with cytotoxic activity from
Artocarpus lanceifolius. Fitoterapia. 73: 668-673.
Ilyas, A., 2013. 2-arylbenzofuran and stilben compounds
from methylene chloride (CH
2
Cl
2
) Extract Leaves of
Artocarpus fretessi hassk. Teknosains Journal.
Jayasinghe, L., Bolasooriya, B.A.I.S., Padmini, W.C.,
Hara, N., Fujimoto, Y., 2004. Geranyl chalcone
derivates with antifungal and radical scavenging.
Phytochem. 65: 1287-1290.
Khan, M.R., Omoloso, A.D., Kihara, M., 2003.
Antibacterial activity of Artocarpus heterophyllus.
Fitoterapia. 74 (5): 501-505.
Ko, H.H., Lu, Y.H., Yang, S.Z., Won, S.J., Lin, C.N.,
2005. Cytotoxic prenylflavonoids from Artocarpus
elasticus. J Nat Prod. 68: 1692-1695.
Kochummen, K.M., 1987. Moraceae in Tree Flora of
Malaya. Vol. 2. Forest Research Institute. Kepong,
Malaysia.
Nguyen, N.T., Nguyen, M.H.K., Nguyen, H.X., Bui,
N.K.N., Majumder, M.T.T.N., 2012. Tyrosinase
inhibitors from the wood of Artocarpus heterophyllus.
J.Nat.Prod. 75, 1951-1995.
Syah, Y.M., Juliawaty, L.D., Achmad, S.A., Hakim, E.H.,
Ghisalberti, E.L., 2006. Cytotoxic isoprenylated
flavones from Artocarpus champeden. Journal
Natural Medicine. 60: 308-312.
Takahashi, M., Fuchino, H., Satake, M., Agatsuma, Y.,
Sekita, S., 2004. In vitro screening of leishmanicidal
activity in Myanmar timber extracs. Biol. Pharm. Bull.
27 (6) 921-925.
Verheij, E.W.M., Coronel, R.E., 1992. Plant Resources of
South Asia No.2 Edible Fruits and Nuts. Prosea
Foundation Bogor.
Wang, Y., Xu, K., Lin, L., Pan, Y., Zheng, X., 2007.
Geranyl-flavonoids from the leaves of Artocarpus
altilis. Phytochemistry. 68, 1300-1306.
Weng, J.R., Chan, S.C., Lu, Y.H., Lin, H.C., Ko, H.H.,
Lin, C.N., 2006. Antiplatelet prenylflavonoids from
Artocarpus communis. Phytochem. 67: 824-829.
Widyawruyanti, A., Subehan., Kalauni, S.K., Awale, S.,
Nindatu, M., Zaini, N.C., Syafruddin, D., Asih, P.B.S.,
Tezuka, Y., Kadota, S., 2007. New prenylated
flavones from Arocarpus champeden, and their
Antimalarial Activity in vitro. J Nat Med. 61: 410-413.
Cytotoxicity of Heterophyllene A, the Derivative of Arylbenzofuran from Stem Bark of Artocarpus calophylla
159
