
Characterization of α-Cellulose from Bagasse Cane Bz 132
(Saccharum officinarum)
Tengku Rachmi Hidayani
1,3
, Basuki Wirjosentono
2*
, Darwin Yunus Nasution
2
and Tamrin
2
1
Postgraduate Chemistry Study Program, Faculty of Mathematics and Natural Sciences, Universitas Sumatera Utara,
Jl. Bioteknologi No. 1 Kampus USU, Medan, Indonesia
2
Department of Chemistry, Faculty of Mathematics and Natural Sciences, Universitas Sumatera Utara, Medan, Indonesia
3
Program Studi Agribisnis Kelapa Sawit, Politeknik Teknologi Kimia Industri, Medan, Indonesia
Keywords: Cellulose, α – Cellulose, Bagasse, Okhamafe Method, Extraction.
Abstract: A study concerning the separation of α - cellulose from bagasse were conducted using Okhamafe. The first
stage is the preparation of bagasse powder type Bz 132. Stage second is the separation of α - cellulose from
bagasse is done with the method Okhamafe done by immersion in 3.5 % HNO acid, bleaching with Sodium
Hypochlorite 1.75 % and purification with 17.5 % NaOH. The third stage is the characterization of α -
cellulose from bagasse types Bz 132 showed visible surface with large round -shaped grains are almost the
same size (uniform). This may indicate that the resulting α - cellulose has a form of homogeneous size (as
large). From the analysis of functional groups with the free OH groups visible wave number 4001.50 cm-1
which shows a typical chain of cellulose CH2OH and OH hydrogen bonds with wave number 3437.30 cm-1
as supporting data. Of the thermal test with test results obtained DTA melting point at a temperature of
60oC and 320oC decomposition point.
1 INTRODUCTION
Sugarcane is one of the agricultural commodities
containing lignocellulose so that it has the potential
as a raw material in the manufacture of
biodegradable composites. So far the use of sugar
cane is still limited to the sugar processing industry
by only taking the water, while the pulp of about 35-
40% of the weight of sugarcane milled is only used
as industrial fuel or may be disposed of so that it
becomes waste (Krisna, 2009). Cellulose is the main
constituent part of woody plant tissue. The
ingredients are mainly found in perennials, however,
cellulose is basically found in every type of plant,
including seasonal plants, shrubs and vines, even the
simplest plants. Such as: mushrooms, algae and
mosses (Tarmansyah, 2007). Separation of α-
cellulose from corn cob fiber was carried out by
Okhamafe by taking fine and dry fibers from corn
cobs which were then immersed in 3.5% HNO3
containing a number of NaNO3 at 900C for 2 hours.
The mixture is then soaked and heated with 2%
NaOH and 2% Sodium Sulfite at 50oC for 1 hour,
then bleached with Sodium hypochlorite
(Ohwoavworhua, 2005). Based on the descriptions
above, the researcher was interested in conducting
research on the extraction of α-cellulose from
sugarcane bagasse Bz 132 (Saccharum officinarum).
The results of α-cellulose from sugarcane bagasse
Bz 132 (Saccharum officinarum) were obtained
analyzed by functional groups by Fourier Transform
Infrared Spectroscopy (FTIR) test, analysis of
morphological properties by Spectra Electro
Magnetic (SEM) test, thermal properties analysis
with Differential Thermal Analysis (DTA) test. This
study is expected to provide information about the
analysis of the properties of α-cellulose from
sugarcane bagasse Bz 132 (Saccharum officinarum)
which is expected to be able to increase the
economic value of bagasse waste.
2 MATERIALS AND METHODS
2.1 Research Location
This research was carried out at the Kimia Terpadu
Universitas Sumatera Utara, the SEM test was
174
Hidayani, T., Wirjosentono, B., Nasution, D. and Tamrin, .
Characterization of -Cellulose from Bagasse Cane Bz 132 (Saccharum officinarum).
DOI: 10.5220/0008864601740177
In Proceedings of the 1st International Conference on Chemical Science and Technology Innovation (ICOCSTI 2019), pages 174-177
ISBN: 978-989-758-415-2
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

conducted at the PPGL Laboratory, the DTA test
was conducted at the Politeknik Teknologi Kimia
Industri (PTKI), the FTIR Test was conducted at the
Bea Cukai Belawan Laboratory.
2.2 Methods
2.2.1 Preparation of Sugarcane Bagasse
Discarded the skin of sugarcane bagasse type Bz
132, milled with a grinder, extraction wet bagasse
from sugarcane waterdir. Wet bagasse washed with
water, soaked in water for 2 hours, dried under the
sun for 6 days, cut to from fine fibers, mashed up
and get dry sugarcane bagasse.
2.2.2 Extraction of α -Celullose from
Bagasse Cane Bz 132
About 75 gram of Bagasse cane put into beakerglass,
added 1000 mL of HNO
3
with 10 mg of NaNO
2
,
dipped for 2 hours in the water bath, washed and
filtrate, residue was heated with 375 mL NaOH 2%
and 375 mL Na Sulfite 2% with temperature 50oC for
one hour, washed and filtrated, residue was heated
with 500 mL sodium hypochlorite 1,75% for 0,5 hour
with temperature 100
o
C for 0,5 hour, washed and
filtrated until neutral pH. Cellulose (residue) added
500 mL NaOH 17,5% and heated for 80
o
C, washed
and filtrated until neutral pH., wet alpha cellulose
dried with oven 60
o
C, and saved in desiccator,
characterization with FTIR, SEM, and DTA test.
3 RESULTS AND DISCUSSIONS
3.1 SEM Analysis of α -Celullose
Bagasse Cane Bz 132 (Saccharum
officinarum)
Figure 1: Test results of α-cellulose SEM from bagasse
pulp Bz 132 (Saccharum officinarum).
Figure 1: is the result of SEM photos of the surface
of α-cellulose from bagasse Bz 132 (Saccharum
officinarum) with a magnification of 1000 times.
Visible round nutiran surface with almost the same
size (uniform). This can show that the resulting α-
cellulose from sugarcane bagasse Bz 132
(Saccharum officinarum) has a homogeneous size.
3.2 the Cross-Linked Al-CMC
The following are the results of α-cellulose FTIR
test from bagasse Bz 132 (Saccharum officinarum).
Figure 2: α-cellulose FTIR Test results from sugarcane
bagasse Bz 132 (Saccharum officinarum).
Table 1: The spectra of FT-IR analysis of α-cellulose from
bagasse Bz 132 (Saccharum officinarum) provide
absorption spectrum peaks with wave numbers.
Functional
group
Wavenumber
Shriner.
(2004)
Free O-H group
4001.50 cm
-1
3500 – 4000
O-H hydrogen
bond
3437.30 cm
-1
3330 – 3500
C-H streching
2898.17 cm
-1
2840 – 3000
C-O carbonil
Cyclic ring
1380.84 cm
-1
897.90 cm
-1
1200 – 1400
800 - 900
From the FTIR spectra of α-cellulose from
sugarcane bagasse Bz 132 (Saccharum officinarum)
it is seen that the OH Group is free with wave
numbers 4001.50 cm
-1
which shows the CH chain
that is typical for hydrogen cellulose and OH bonds
with wave numbers 3437.30 cm-1 as supporting
data. And there is a CH stretching at wave number
2898.17 cm
-1
which proves the existence of CH
bonds at the end of cellulose and carbonyl CO at the
wave number 1642.46 cm
-1
which is also a typical
group of cellulose supported by fingerprint region
finger 897.90 cm
-1
which shows a cyclic ring chain.
So it can be concluded that there are cellulose
compounds in the spectra displayed.
3.3 Result of DTA Test from
α -Cellulose N 132 (Saccharum
officinarum)
The tool used in the DTA test on α-cellulose from
bagasse Bz 132 (Saccharum officinarum) is
termocouple / mv: PR / 15mv brand shimadzu,
japan. The temperature of the experiment is the
Characterization of -Cellulose from Bagasse Cane Bz 132 (Saccharum officinarum)
175
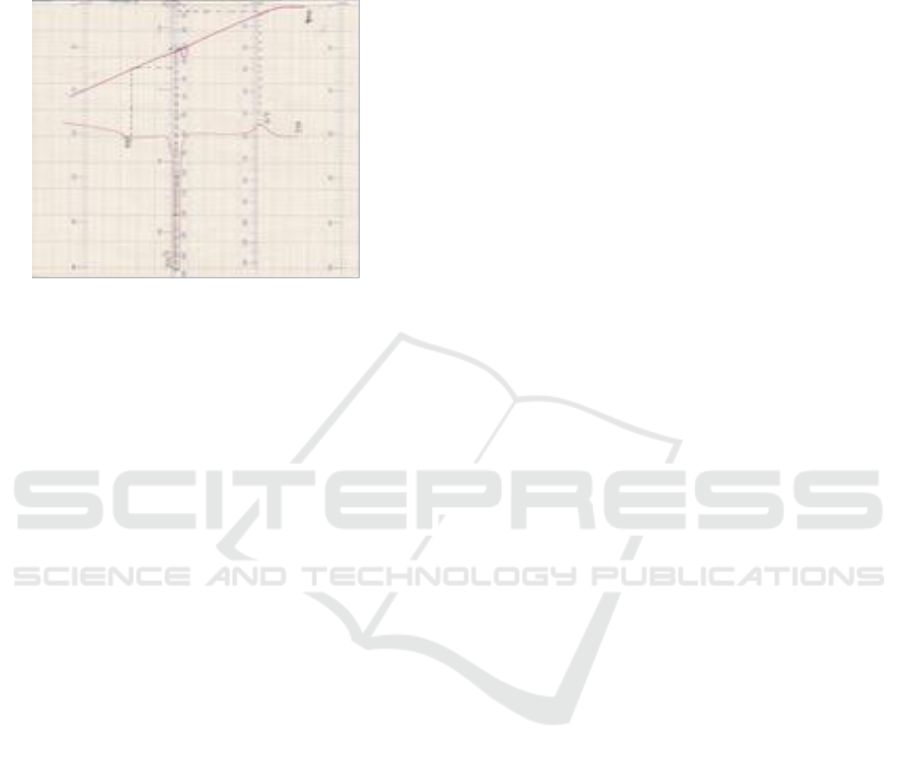
temperature of 20°C - 600°C. MCC used in this test
is 30 gram with DTA range ± 250 µv, heating speed
10°C mm / minute and chard speed 2.5 mm / minute.
The following is a picture of the DTA test
equipment used.
Figure 3: DTA Test.
From thermocouple DTA, α-cellulose from
sugarcane bagasse Bz 132 (Saccharum officinarum)
shows a peak at 60°C, a peak formed on the right
area which shows a decrease in temperature
(endotherm) and a peak on the left at 320°C
indicating there is an increase in temperature
(exotherm). At 60°C α-cellulose from bagasse Bz
132 (Saccharum officinarum) evaporates which is
likely to be the water that is still stored α-cellulose
from bagasse Bz 132 (Saccharum officinarum), and
at 320°C α-cellulose from pulp sugar cane Bz 132
(Saccharum officinarum) burns by showing its
optimum peak.
4 CONCLUSIONS
Separation of α-cellulose from bagasse is done by
the Okhamafe method by extraction which is done
by immersion in 3.5% of HNO
3
acid, bleaching with
Sodium Hipochlorite 1.75% and purification with
NaOH 17.5%. Analysis of the characteristics of the
resulting α-cellulose was obtained from the results
of α-cellulose SEM photos of the surface with a
magnification of 1000 times. Visible spherical-
shaped surfaces with almost the same size (uniform).
This can indicate that the resulting α-cellulose has a
homogeneous size (equal in size). From the
functional group analysis, we can see the free O-H
group with wave number 4001.50 cm
-1
which shows
the CH2OH chain that is typical for cellulose and O-
H hydrogen bonds with wave numbers 3437.30 cm
-1
as supporting data. From the thermal test, the DTA
test obtained the melting point at a temperature of
60
0
C and a decomposition point of 320
0
C according
to the reference cellulose in general.
ACKNOWLEDGEMENTS
This research was carried out funded by Politeknik
Teknologi Kimia Industri Medan.
REFERENCES
Astawan, M., 2008. Kemasan:Pengaman Dan Pengawet
Makanan. Senior:Jakarta
Azubuike, Chukweumeka.P.C., Okhamafe, Augustine. O.,
Falodun, abiodun., 2011. Some Pharma Copocial and
diluent-Binder Properties of MaizeCob-derive from α-
cellulose in Selected Tablet Formulations. Journal of
Chemical and Pharmaceutical research. 481-488.
Eichhorn, S. J., Baillie, C. A., Zafeiropoulos, N.,
Mwaikambo, L. Y., Ansell, M. P., Dufresne, A.,
Entwistle, K. M., Herrera-Franco, P. J., Escamilla, G.
C., Groom, L. and Hughes, M., 2001. Current
international research into cellulosic fibres and
composites. Journal of materials Science, 36(9),
pp.2107-2131.
Kazakova, E. G. and Demin, V. A., 2009. A new procedure
for preparing microcrystalline cellulose. Russian Journal
of Applied Chemistry, 82(3), pp.496-499.
Malau, KM., 2009. Pemanfaatan Ampas Tebu Sebagai
Bahan Baku Dalam Pembuatan Papan Partikel.Skripsi.
Medan: USU.
Leppänen, K., Andersson, S., Torkkeli, M., Knaapila, M.,
Kotelnikova, N. and Serimaa, R., 2009. Structure of
cellulose and microcrystalline cellulose from various
wood species, cotton and flax studied by X-ray
scattering. Cellulose, 16(6), pp.999-1015.
Ohwoavworhua, F. O. and Adelakun, T. A., 2005. Some
physical characteristics of microcrystalline cellulose
obtained from raw cotton of Cochlospermum
planchonii. Tropical Journal of Pharmaceutical
Research, 4(2), pp.501-507.
Ohwoavworhua, F. O. and Adelakun, T. A., 2005.
Phosphoric acid-mediated depolymerization and
decrystallization of α-Cellulose obtained from corn
cob: preparation of low crystallinity cellulose and
some physicochemical properties. Tropical Journal of
Pharmaceutical Research, 4(2), pp.509-516.
Oksman, K., 2007. Extrusion and Mechanical Properties
of Highly Filled Cellulose Fibre-Polypropylene
Composites. Division of Manufacturing and Design of
Wood and Bionano composites. Lulea University of
Technology: Sweden.
Penebar Swadaya., 2000. Pembudidayaan Tebu Dilahan
Sawah dan Tegalan.Penebar Swadaya: Jakarta.
ICOCSTI 2019 - International Conference on Chemical Science and Technology Innovation
176

That, T., 2008. Improving the Processing and Properties of
Cellulose fibre Composite. National Research
Council: Canada.
Tarmansyah, U. S., 2007. Pemanfaatan Serat Rami Untuk
Pembuatan Selulosa. STT No.2288.Volume 10 Nomor
18. Tim Puslitbang Indhan Balitbang Dephan: Jakarta.
Characterization of -Cellulose from Bagasse Cane Bz 132 (Saccharum officinarum)
177
