
Antioxidants Activity of the Kecombrang Flower (Etlingera elatior)
Extract by using 1,1-diphenyl-2-picrilhidrazyl (DPPH) Method
Maulidna
1,3
, Basuki Wirjosentono
2*
, Tamrin
2
and Lamek Marpaung
2
1
Postgraduate Chemistry Study Program, Faculty of Mathematics and Natural Sciences, Universitas Sumatera Utara,
Jl. Bioteknologi No. 1 Kampus USU, Medan, Indonesia
2
Department of Chemistry, Faculty of Mathematics and Natural Sciences, Universitas Sumatera Utara, Medan
3
Politeknik Teknologi Kimia Industri, Medan, Indonesia
Keywords: Antioxidant, Flower Extract Kecombrang, DPPH, Secondary Metabolite, Alkaloids.
Abstract: Kecombrang (Etlingera elatior) is a zingiberaceae plant, which has long been known as one of the vegetables
and used by the community as a nutritious food to preserve food because of the active substances contained
in it, such as saponins, flavanoids, and polyphenols. In this research, antioxidants activity of kecombrang
flower extract was measured by using 1,1-diphenyl-2-picrilhidrazil (DPPH) method. The kecombrang flower
extract has several secondary metabolite compounds, such as alkaloid, terpenoid, steroid, phenolic, flavonoid,
tanin with very small antioxidant activity, which is showed by inhibition percentage. As many as 100 ppm
concentration of the kecombrang flower extract, ie, methanol extract 3.21%, ethyl acetate extract 5.08 %, and
n-hexane extract 30.29%. So it can be concluded that the antioxidant activity of flower kecombrang (Etlingera
elatior) is less active.
1 INTRODUCTION
Human health is very dependent on the environment
nowadays. Besides, the environment which is full of
pollutants, will be able to poison the body either
through air or food. One of the causes of
environmental pollution is pesticide residue. It has
been reported that pesticide residues can cause the
diseases because of the presence of radical
compounds that can oxidize cells (Zakaria et al,
1996). Excessive cell oxidation process will lead to
many diseases, such as cancer, diabetes, heart disease
(Fajriah et al, 2007) atherosclerosis, cataracts and
premature aging (Langseth et al, 2000). The body
itself produces antioxidants that can reduce the
negative effects of free radical reactions. As long as
the balance between free radicals and endogenous
antioxidants is maintained, the adverse effects of free
radicals can be neutralized (Subarnas et al, 2001).
Various diseases caused by radical compounds are
growing, this makes the research continue to be done
as an effort to be able to find substances as drugs that
can play an active role in preventing and overcoming
radical compounds in the body reported that there are
two types of drugs that can be used as an alternative
treatment of synthetic drugs and traditional medicine
in the world of drugs. Besides, choosing a drug should
consider as the side effects of drug performance.
Drugs from natural ingredients have relatively fewer
side effects compared to synthetic drugs (Utami et al,
2008). Generally, Indonesian prefer to take the
natural-made medicines in the effort to prevent and
treat an attack of disease by drinking water extract
from certain plants or by attaching the extract to the
sick body part (Mohd Jaafar et al, 2008).
Free radical compounds can be overcome by a free
radical prophylactic called antioxidants. The
antioxidant is a component capable of inhibiting
nucleic acid, lipid oxidation by initiation or
propagation of chain oxidation reactions.
Antioxidants can protect the body from various
degenerative diseases in accordance with the main
function of antioxidants namely, neutralizing free
radical compounds (Winarsi et al, 2007), reducing
agents, free radical damping and metal pro-oxidant
complex (Poerawinata M, et al, 2007). The isolated
antioxidant compounds contained in high plants are
ß-carotene, vitamin C, vitamin E, flavonoids,
curcuminoids and polyphenol compounds
(Alamendah's et al, 2013).
Based on the above explanation, this study aims to
conduct the research on kecombrang plant, which is
Maulidna, ., Wirjosentono, B., Tamrin, . and Marpaung, L.
Antioxidants Activity of the Kecombrang Flower (Etlingera elatior) Extract by using 1.1-diphenyl-2-picrilhidrazyl (DPPH) Method.
DOI: 10.5220/0008869301970203
In Proceedings of the 1st International Conference on Chemical Science and Technology Innovation (ICOCSTI 2019), pages 197-203
ISBN: 978-989-758-415-2
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
197

believed to have antioxidant activity as concluded by
Tan et al.. Regarding to the activity of chewing as an
antioxidant, testing of antioxidant kecombrang can be
done by using of the DPPH method. The DPPH
method is low-cost and easy-to-prepare method
where the DPPH acted as free radical will react with
antioxidant compound so that the color of test
solution changes from purple to yellow (Pratama et
al, 2015).
2 MATERIALS AND METHODS
2.1 Materials
The instruments used are UV-Vis Spectrophotometer,
vacuum rotary evaporator, separator funnel,
sonicator, incubator, analytical scales, dilution bottle,
vial bottle. The ingredients used are flower samples
of coconut, methanol solvent, Ethyl acetate solvent,
n-Hexane solvent, DPPH powder, and methanol p.a.
2.2 Method
2.2.1 Sample Preparation
The sample was obtained from one of the areas in Deli
Serdang with a weight of 2 Kg, which then dried and
graded into small size and weighed the sample
weight.
2.2.2 Maceration
Samples that have been scaled to a small size, are
inserted into glass bottles and methanol solvent added
until the sample portion is completely submerged.
Subsequent samples in glass bottles were left
submerged for 2 x 24 h. After 2 x 24 h the sample is
filtered and then the filtrate of the sample is floated in
a clean glass bottle.
2.2.3 Vacuum Rotary Evaporator
The sample filtrate has been accommodated, then
inserted into the rotavapor flask according to the
pumpkin container, and subsequently in the rotary
vapor until the filtrate becomes thickened thick and
then allowed to evaporate until the viscous filtrate,
then repeated rotary evaporator by incorporating
other filtrate.
2.2.4 Separation of Extract with Separating
Funnel
The viscous filtrate was taken slightly and diluted
with some mL of methanol slowly, then inserted into
the separating funnel and added with several mL of n-
hexane solvent, subsequently separated funnel for a
few seconds until the second solvent was evenly
mixed and then placed the separating funnel in the
stem neck stative until both solvents are perfectly
separated. Further separated layers of methanol with
a layer of n-hexane and each of them accommodated
in glass bottles.
2.2.5 Vacuum Rotary Evaporator Methanol
Extract and N-hexane Extract
Each filtrate of methanol extract and filtrate of n-
hexane extracts were regenerated until each filtrate
changed to blackish, and then allowed to dry until a
thickened extract was formed.
2.2.6 Separation of Methanol Condensed
Extract with Ethyl Acetate Solvent
The methanol condensed extract was extracted by
adding the ethyl acetate solvent until the viscous
portion of the methanol extract was insoluble in the
ethyl acetate solvent.
2.2.7 Calculation of Yield Percentage
Each extract that has been produced then calculated
the yield by using the following formula:
(1)
2.3 Characterization
2.3.1 Phytochemical Screening
The three extracts have been obtained, phytochemical
screening such as the identification of alkaloids,
terpenoids, steroids, phenolics, flavonoids, saponins,
quinones, and tannins. The alkaloid test was
performed by reacting the extract with a Dragendroff
reagent and producing a brown red and orange
(Robinson et al, 1995). The terpenoid and the steroid
tests are performed by reacting the extract with
Lieberman-Burchard reagent and producing a pink or
purple color (Harborne et al, 1987). Flavonoid test
was done by dissolving the extract in water and then
added Mg powder and the added concentrated HCl
and shaken strong, positive test by red, orange, or
purple (Robinson et al, 1995). The saponin test is
ICOCSTI 2019 - International Conference on Chemical Science and Technology Innovation
198
done by dissolving the extract in water and then
shaking strongly for a few seconds and will cause a
stable foam, then added 1% HCl if the foam arises
with a height of 1-3 cm and persist for 15 minutes
indicating the presence of saponins (Harborne et al,
1987). Phenolic test is performed by reacting the
extract with 1% iron and 1% chloride reagent and
positive when it gives strong green, red, violet, blue
and black (Erwinsyah et al, 2016). Tannin test is done
by dissolving the extract in water and then the added
a few drops of iron reagent (III) chloride 1% positive
result when giving rise to dark blue, green (LIPI,
2017). The quinone test is performed by diluting the
extract into water and added a few drops of positive
1N NaOH by causing a red color (LIPI, 2017).
2.3.2 The Antioxidant Activity Test
The antioxidant activity is determined by free radical
damping method using the DPPH. Weighed the
DPPH powder as much as 2.4 mg and dissolved with
methanol as much as 15 mL and then placed in a dark
bottle. As many as 0.4 mM the DPPH solution was
pierced 1 mL and inserted in a 5 mL scale reaction
tube, then the added methanol pro analysis to the
boundary marker, and the tube was covered with
aluminum foil then homogenized.
Weighed each viscous the extract of 5 mg was
used an analytical scale, then dissolved into 10 mL
the methanol pro analysis, the solution is the parent
liquor. Then the parent solution was piped into the
test tube with a volume of 5 mL of 1000 μL to obtain
a concentration of 100 ppm.
A total of 3 mg of the vitamin C was weighed and
then dissolved with the methanol p.a up to 10 mL.
Furthermore, the 150, 117, 83, 50, and 17 μL pipes
were inserted into a tube of scale covered with
aluminum foil and then added 1 mL of 1 mM the
DPPH solution and added the methanol pa to 5 mL
boundary marker on the scale tube to obtain
concentration 1, 3 , 5, 7, 9 ppm.
The test solution with a concentration of 100 ppm
was incubated with 37
o
C for 30 minutes. Subsequent
absorption of the solution was measured at a
maximum absorption wavelength of 517 nm by using
a visible light spectrophotometer.
Formal absorption, positive control and
absorbance of the test solution measured on the UV-
Vis spectrophotometer and recorded by entering the
absorption results percentage inhibitor in the
following formula:
(2)
3 RESULTS AND DISCUSSION
3.1 Results
The results of research conducted at Bioproses
Laboratory Politeknik Teknologi Kimia Industri
Medan can be seen in Table 1; 2; 3; and 4 below:
Table 1: Value of Randemen Percentage of Kecombrang
Flower (Etlingera elatior) Extract.
Extract
Weight Extract
(g)
Yield
(%)
n-hexane
10.21
0.51
Ethyl acetate
2.41
0.12
Methanol
1.45
0.07
Table 2: Phytochemical Screening Results of Kecombrang
Flower (Etlingera elatior) Extract.
Methanol
Extract
Ethyl
Acetate
Extract
n-
hexane
Extract
Alkaloid
-
+
+
Terpenoid
+
-
+
Steroid
+
-
-
Phenolic
+
+
-
Flavonoid
-
+
-
Saponin
-
-
-
Quinon
-
-
-
Tannin
+
+
+
Table 3: Value of Inhibitor percentage of the Kecombrang
Flower Extract (Etlingera elatior) at 100 ppm
Concentration.
Extract
Concentration
Absorbance
%
inhibition
n-hexane
100
0.672
30.29
Ethyl
Acetate
100
0.915
5.08
Methano
l
100
0.933
3.21
Blank
100
0.964
-
Table 4: Antioxidant Activity Test Results of Vitamin C.
Concentration
(ppm)
Absorbance
%
inihibition
IC
50
(ppm)
1
0.896
7.05
4.51
3
0.725
24.79
5
0.406
57.8
7
0.066
93.15
9
0.024
97.51
Antioxidants Activity of the Kecombrang Flower (Etlingera elatior) Extract by using 1.1-diphenyl-2-picrilhidrazyl (DPPH) Method
199
3.2 Discussions
3.2.1 Extraction
The samples from flowering plants were cut into
small pieces and dried, then the dried samples were
macerated using organic solvent, methanol solvent
for 2 x 24 h with 4 repeats of maceration until the
solvent became translucent until the secondary
metabolite was no longer soluble in the methanol
solvent. Maceration is a technique of immersion to
the material to be extracted. Samples that are small in
size are immersed in organic solvents for some time
and then filtered and the result is a filtrate (Sitorus et
al, 2010). The purpose of maceration so that the
secondary metabolite compounds contained in the
sample can be dissolved in the solvent so that it can
be obtained filtrate sample flowers kecombrang.
Maceration uses organic solvents, the function of
organic solvents to penetrate the cell wall of the
sample and into the cell cavity resulting in the
secondary metabolite compounds contained in the
cell to dissolve. The dissolution of secondary
metabolite compounds is due to the difference in
concentration between the secondary metabolite
compounds and the organic solvent resulting in
diffusion. After the diffusion process takes place, the
sample filtrate is accommodated into a glass for the
rotary evaporator process.
In the rotary evaporator, the filtrate concentration
process of the kecombrang flower sample is based on
the vapor pressure of the solvent influenced by the
temperature. The concentration process uses a
vacuum pump with a cooling water stream so that the
solvent present in the apparatus will decrease the
vapor pressure. When the solvent vapor pressure drop
is equal to the atmospheric pressure the solvent will
boil, so that the solvent present in the sample filtrate
will be able to evaporate faster at a temperature below
its boiling point. This process is done until the extract
obtained thick flowers kecombrang.
The obtained flower extract of the resulting
kecombrang was then extracted with a n-hexane
solvent in order for the non-polar secondary
metabolite compounds to be separated by a polar one.
The extract of thick flower extract of kecombrang
was done by using separating funnel tool so that
separation of secondary metabolite compounds with
different polarity properties will be seen clearly with
the formation of 2 layers where the top layer is n-
hexane solvent while the bottom layer is methanol
solvent. Each flower extract that will be extracted in
a separating funnel is dissolved first with a methanol
solvent so that the extract becomes more dilute and at
the time of added n-hexane solvent will be easier
separation process. After separation by using
separating funnel, both layers of flower extract of
kecombrang are accommodated in glass bottles'.
Furthermore, the two flower extracts concentrated by
using a rotary evaporator. The concentrated methanol
extract of the kecombrang flower was further
extracted with ethyl acetate solvent by adding ethyl
acetate solvent in the methanol concentrated extract
until the methanol extract of the flower kecombrang
was insoluble in the ethyl acetate solvent, then the
ethyl acetate extract of the flower kecombrang was
left alone the solvent evaporated. The result of extract
process was obtained three extracts from kecombrang
interest that is methanol extract, ethyl acetate extract,
and n-hexane extract then calculated% yield,
phytochemical screening, calculation of DPPH
damping resistor.
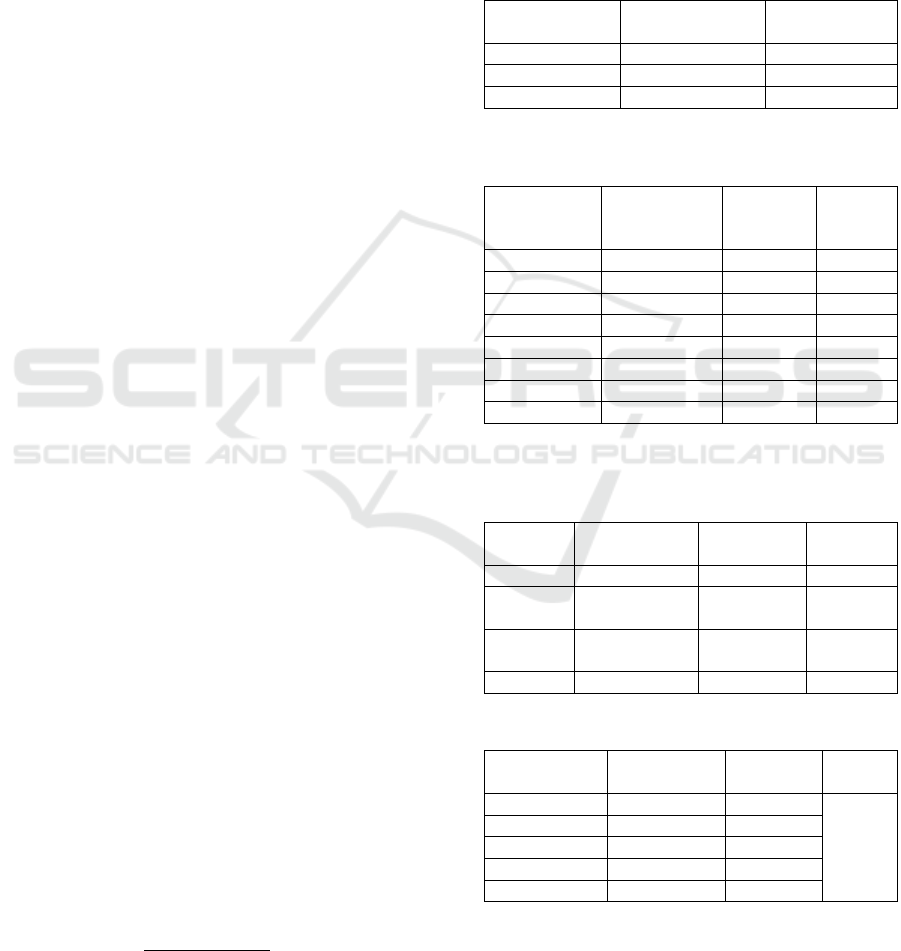
Figure 1: Value of Randemen Percentage of Kecombrang
Flower (Etlingera elatior) Extract.
The graph above illustrates the percentage of yield of
kecombrang flower (Etlingera elatior) extract. The
extract was done yield percentage calculation with
the aim to know how many extracts will be obtained
from so many samples was used, so to conduct further
research will be easy to predict how many samples
will be extracted to get the extract according to
requirement. Randemen percentage were extracted
methanol 1.45 g with percentage of 0.07%, ethyl
acetate extract 2.41 g with randemen percentage
0.12% and n-hexane extract of 10.21 g with 0.5%
yield percentage.
ICOCSTI 2019 - International Conference on Chemical Science and Technology Innovation
200

3.2.2 The Phytochemical Screening
The three extracts that have been calculated
rendemen percentage phytochemical screening. The
purpose of phytochemical screening is to know the
type of secondary metabolite compounds contained in
the sample. The method was used because easier and
simpler to do it.
Figure 2: Phytochemical Screening Results of the Extract.
3.2.3 Kecombrang Flower (Etlingera elatior)
The graph above describes the results of
phytochemical screening of the kecombrang flower
extract (Etlingera elatior). Based on the Fig. 2. can be
explained that the extract of n-hexane found the
secondary metabolite content of alkaloids,
terpenoids, and tannins. Ethyl acetate extract of
secondary metabolite content in the form of alkaloids,
phenolics, flavonoids, and tannins. While the extract
methanol terpenoid content, steroids, fennolik and
tannins. The results of screening of studies that have
been performed with similar extracts from bogor
areas indicate the presence of secondary metabolites
from ethanol extracts of alkaloids, flavonoids,
saponins, tannins, steroids and terpenoids, ethyl
acetate extracts of alkaloids, flavonoids, steroid and
terpenoidal saponins, and n-hexane extract not
detected secondary metabolite content (Verawati et
al, 2014). The content of secondary metabolite of
kecombrang flower extract from Deli Serdang area
with extract of kecombrang flower from Deli Serdang
area has difference such as ethanol extract of
kecombrang flower from Deli Serdang area detected
alkaloid and falvonoid content and not found in
methanol extract of kecombrang flower from Tanah
Karo area. This difference in detectable secondary
metabolite content may be caused by the precursor of
the biosynthesis of secondary metabolite formation as
well as the texture of the soil in which the flower
derived origin (Verawati et al, 2014). (Milana et al,
2016) also reported that the formation of secondary
metabolites is strongly influenced by soil nutrients
having linear relationships such as nitrogen,
potassium, organic matter and carbon.
3.2.4 Test of Antioxidant Activity by using
the DPPH Method
The antioxidant activity test was performed to see the
bioactivity of the kecombrang flower by looking at
the percentage value of the sample inhibitory power.
To determine the inhibitory power of the sample as
antioxidant can be done by using the DPPH method.
The antioxidant activity test used the DPPH method
because it is easier to do and not expensive.
Testing of antioxidant activity using 0.4 mM
DPPH solution made by weighing DPPH powder as
much as 2.4 mg and dissolved into 15 mL methanol
pa. Further weighed the extract to be tested as much
as 5 mg and dissolved in a solution of methanol pa 10
mL and disonikasi so that the solution becomes
homogeneous. Test solution was then prepared with
concentration of 100 ppm by pipette 1 mL of DPPH
solution into three scale test tubes wrapped with
aluminum foil and added extract solution on each
tube as much as 1 mL then added methanol pa to 5
mL scale and for blank DPPH solution in 5 mL test
tube. The test tube was then detoxified for 30 minutes
to speed up the reaction between samples acting as
antioxidants with DPPH free radicals. After 30
minutes the test solution is ready for measurement by
a UV-Vis spectrophotometer to determine the
absorbance of each test solution.
Testing with UV-Vis spectrophotometer was
performed at 517 nm wavelength because at that
wavelength had the optimum absorbance ability for
antioxidant activity test. Furthermore, the
measurement of the absorbance of each test solution
in duplicate.
For methanol extract test solution obtained
absorbance 0.933, and for ethyl acetate extract
obtained absorbance of 0.915, while extract n-Hexan
obtained absorbance of 0.672, and for blank obtained
absorbance 0.964.
Antioxidants Activity of the Kecombrang Flower (Etlingera elatior) Extract by using 1.1-diphenyl-2-picrilhidrazyl (DPPH) Method
201
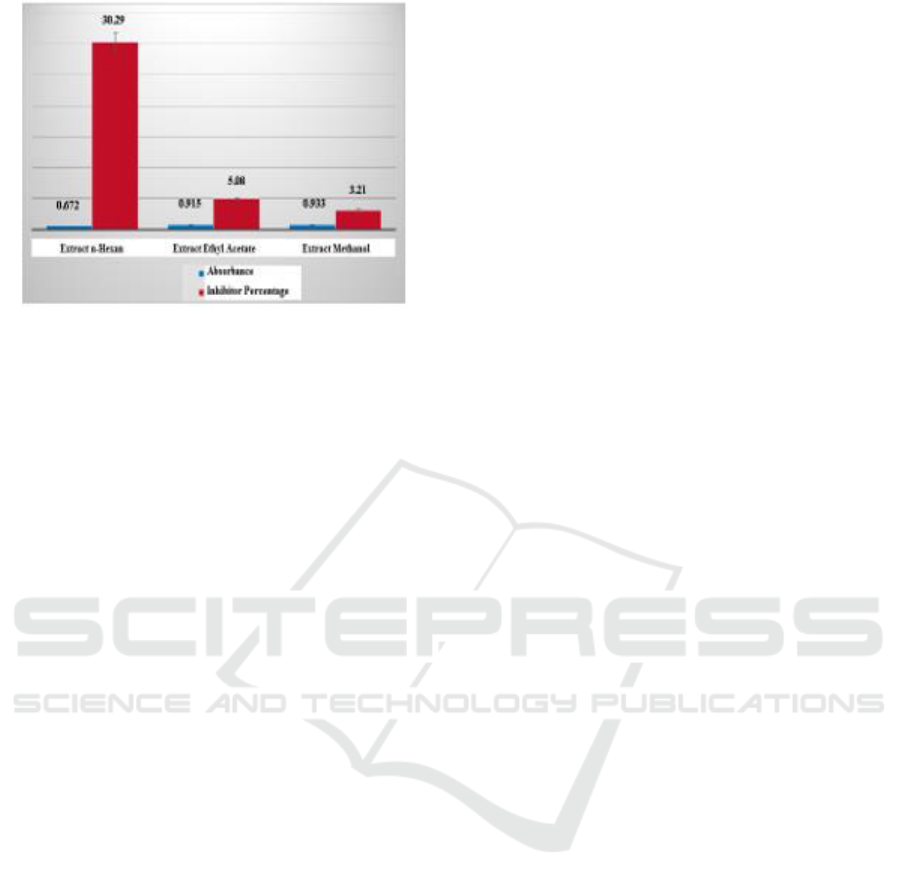
Figure 3: Value of Inhibiton Percentage of Kecombrang
Flower Extract (Etlingera elatior) at 100 ppm
Concentration.
After calculation can be obtained percentage of the
inhibitory of each-tipa extract. The extract of n-
hexane has an inhibitory percentage of 30.29%, ethyl
acetate extract has 5% inhibition of 5.08% and
methanol extract has an inhibitor percentage of
3.21%, so it can be said that the antioxidant activity
of the three extracts are very small. (Anisa et al, 2014)
says antioxidant activity can be expressed as IC50
(Inhibition Concentration fifty) if concentration to
free radical clearance is 50%. Vitamin C test results
have very good antioxidant activity and obtained
IC50 4.51 ppm, while the results of calculation of the
inhibitory of the three extracts are not up to 50%,
Therefore the three extracts in the test beforehand in
a concentration of 100 ppm in order to know whether
the extract has activity 50 good percentage or not
before IC50 measurement.
4 CONCLUSIONS
Based on the data of the research, it can be concluded
that the sample extract of kecombrang flowers has
some secondary metabolite compounds such as
Alkaloid, Terpenoid, Steroid, Phenolic, Flavonoid,
Tanin. In addition, the extract of kecombrang flower
sample also has very small antioxidant activity at
concentration of 100 ppm that is, methanol extract
3.21%, etil acetate extract 5.08%, and 30.29% n-
hexane extract, so it can be concluded that the
antioxidant activity of flower kecombrang (Etlingera
elatior) less active.
ACKNOWLEDGEMENTS
This work had been supported by Politeknik
Teknologi Kimia Industri Medan. The authors would
like to thank the very useful suggestion from the
editors and reviewers.
REFERENCES
Alamendah’s Blog. 2009. Tingkat pencemaran Udara di
Indonesia. https://alamendah.org/2009/09/23/tingkat-
pencemaran-udara-di indonesia/. Tanggal akses 12
November 2017.
Erwinsyah, 2016. Skrining Fitokimia dan Uji Aktivitas
Antioksidan Ekstrak Benalu yang Tumbuh pada
Tanaman Benalu Bungur (Scurrula ferruginea (Jack)
Danser) Dengan Metode DPPH (1.1-difenil-2-
pikrilhidrazil). Universitas Mulawarman, Samarinda.
Fajriah S, Akhmad Darmawan, Andini Suridowo dan Nina
Artanti. 2007. Isolasi Senyawa Antioksidan dari
Ekstrak Etil Asetat Daun Benalu (Dendrophthoe
petandra L) yang Tumbuh pada Inang Lobi-Lobi
Kawasan Puspitek, Serpong: Pusat Penelitian Kimia
Lembaga Ilmu Pengetahuan Indonesia.
Harborne, J. B. 1987. Metode Fitokimia Penuntun Cara
Modera Menganalisis Tumbuhan. Terjemahan: Kosasih
P, Soediro iwang, Bandung ITB.
Jackie et al. BMC Research Notes. 2011.Antioxidant
effects of Etlingera elatior flower extract against lead
acetate - induced perturbations in free radical
scavenging enzymes and lipid peroxidation in rats.
https://bmcresnotes.biomedcentral.com/articles/10.118
6/1756-0500-4-67. Tanggal akses 12 November 2017.
Langseth, L. 2000. Antioxidants and Their Effect on
Health. Essentials of Functional Foods. Aspen
Publication, Gainthersbur
Lipi, 2014. Bioteknologi Untuk Kehidupan Lebih Baik.
http//www.biotek.lipi.go.id. Tanggal akses 29
Novmber 2017.
Mohd Jaafar, Faridahanim et al. 2007. Analysis of Essential
Oils of Leaves, Stems, Flowers, And Rhizomes Of
Etlingera Elatior (JACK) R. M. Smith.The Malaysian
Journal of Analytical Scienses. Universiti Teknologi
MARA. Kuala Lumpur.
Naufalin Rifda and Herastuti Sri Rukmini. 2016.
Antioxidant Activity and Physicochemical Properties
of Nicolaia speciosa Flower extract.Agriculture and
Agricultural Science Procedia 9. Jendral Soedirman
University. Purwokerto.
Rifda, Naufalin. Antimicrobial Activity of Fruit Peel
Kecombrang Formula (Nicolaia speciosa HORAN) As
Natural Preservative. Program Studi Ilmu dan
Teknologi Pangan. Universitas Jenderal Soedirman.
Purwokerto
Poerawinata M. 2007 Uji Aktivitas pada Daun Pandan
(Pandanus polycephalus). skripsi. Universitas
Indonesia, Depok.
ICOCSTI 2019 - International Conference on Chemical Science and Technology Innovation
202

Pramata, Galih Putra. 2015. Identifikasi Senyawa Aktif
Antioksidan dalam Tanaman Selada Air (Nasturtium
officinale R. Br). Skripsi. Universitas Pancasila,
Jakarta.
Robinson, T. 1995. Kandungan Organik Tingkat Tinggi.
Bandung: Institut Teknologi Bandung.
Subarnas, A. 2001. Komponen Aktif Antioksidan dalam
Bahan Alam, Seminar Nasional dan Lokakarya
Pemahaman dan Konsep Radikal Bebas dan Peranan
Antioksidan dalam Meningkatkan Kesehatan Menuju
Indonesia Sehat 2010. Pusat Penelitian Padjadjaran: 28-
40.
Utami N., dan Robbara. M. 2008. Identifikasi Senyawa
Alkaloida dari Ekstrak Heksana daun Argratum
conyzoides. Lin. Prosiding Hasil Penelitian Unila.
Antioxidants Activity of the Kecombrang Flower (Etlingera elatior) Extract by using 1.1-diphenyl-2-picrilhidrazyl (DPPH) Method
203
