
Synthesis of Quatenary Ammonium Compounds from Eugenol
through Mannich and Methylation Reactions
and Its Antibacterial Activity
Sabarmin Perangin-angin* and Sri Wahyuni Barus
Departement of Chemistry, Faculty of Mathematics and Natural Sciences, Universitas Sumatera Utara, Medan, Indonesia
Keywords: Eugenol, Mannich Reaction, Methylation, Antibacterial,
6-[(N-iodo-N-methyl-N-methyl-N-methylamino)methyl]-4-alyl-2-methoxy phenol.
Abstract: 6-[(N-iodo-N-methyl-N-methyl-N-methylamino)methyl]-4-alyl-2-methoxyphenol was synthesized through
Mannich and methylation reaction. Mannich reaction was carried out by reacting eugenol, formaldehyde
37%, and dimethylamine 40% with ethanol at temperature of 78°C for 90 minutes and 4-allyl-6-
(dimethylamino)methyl-2-methoxyphenol was obtained with yield of 83,03%. Quartenary ammonium salt,
6-[(N-iodo-N-methyl-N-methyl-N-methylamino)methyl]-4-allyl-2-methoxyphenol, was obtained through
methylation reaction of 4-allyl-6-(dimethylamino)methyl-2-methoxyphenol with methyl iodide in ethanol.
In the FTIR spectrum, the specific peak of ammonium salt was exhibited at 948.98 and 455.20 cm-1. The
antibacterial activity of 6-[(N-iodo-N-metil-N-methyl-N-methylamino)methyl]-4-allyl-2-methoxyphenol
was performed against E. coli and S. aureus, this quaternary ammonium salt exhibited a strong activity.
1 INTRODUCTION
Eugenol is the main component contained in clove
oil (Syzygium aromaticm), it can reach 70-96%.
Therefore, the quality of clove oil is depended on the
eugenol content, the increase of eugenol content can
influence its quality and price. Eugenol has several
functional groups, i.e. hydroxyl (-OH), allyl (-CH
2
-
CH=CH
2
) and methoxy (-OCH
3
). These functional
groups can be chemically modified to be its derivate
which varies bioactivities. Eugenol has been known
as an important precursor for synthesizing a
particular compound with specific bioactivity
(Towaha, 2012).
Many researches that focused in eugenol has
been carried out, especially for synthesizing eugenol
derivatives, such as the transformation of allyl
groups in eugenol into aldehyde groups, that can be
found in vanillin which already used as food
additives. The other is the transformation of
hydroxyl groups into alkyl, acyl or acetyl, that can
be found in methyl eugenol, eugenol benzoate and
acetyl eugenol (Sastrohamidjojo, 2004).
Amine derivative compounds, such as halide
quaternary ammonium salt (R
4
N
+
X
-
), can be used in
wide area. Quaternary ammonium salt can act as a
phase transfer catalyst. This catalyst is widely used
for heterogeneous reactions involving ionic species
in non-polar solvents which cannot dissolve ionic
species. Ionic species are commonly found in the
liquid phase, while the compounds that will be
reacted are found in the organic phase and both
species cannot mix each other. The quaternary
ammonium salt can also act as a surfactant.
Surfactants reduce the surface tension of water by
breaking hydrogen bonds on the surface. Most
surfactants containing nitrogen bases are cationic
surfactants (Perangin-angin, 2002).
Quaternary ammonium salt can be used as an
antibacterial (Stanley, 2011). Antibacterial are
substances that can inhibit the growth of bacteria by
disrupting the microbial metabolism. Antibacterial
can only be used if they have selective toxicity, it
means they can kill bacteria that cause disease, but
they are not toxic to human. The mechanism of
antibacterial compounds can be divided into a)
inhibiting cell wall synthesis, b) disturbing the cell
wall permeability, c) inhibiting the specific enzyme,
and d) inhibiting the synthesis of nucleic acids and
proteins. Cytoplasm of all living cells are covered by
the cytoplasmic membrane that acts as a barrier with
selective permeability to carry out as active transport
Perangin-angin, S. and Wahyuni Barus, S.
Synthesis of Quatenary Ammonium Compounds from Eugenol through Mannich and Methylation Reactions and Its Antibacterial Activity.
DOI: 10.5220/0008879202230228
In Proceedings of the 1st International Conference on Chemical Science and Technology Innovation (ICOCSTI 2019), pages 223-228
ISBN: 978-989-758-415-2
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
223

arrangement. If the permeability of the cytoplasmic
membrane function is disrupted by substances, i.e.
surfactant, the permeability of the cell wall will
change or even become damaged (Madigan, 2005).
The Mannich reaction is a reaction to modify
enolate compounds to be dialkylamino methylene
that have active hydrogen atoms. This reaction
occurs through a condensation reaction on
functionals groups that can be enolized with
imminium ions (Mannich base), i.e. a product from
the reaction between formaldehyde and secondary
amines (Purwono and Daruningsih, 2010). In
(Karanov et al., 1995), the synthesis of 2-methoxy-
4-(1-prophenyl)-6-phenol through Mannich reaction
with eugenol, formaldehyde, and amine compound
showed an activities as plant growth control. In the
previous work, (Perangin-angin, 2002) performed a
synthesis reaction between eugenol and dimethyl
sulphate in alkaline condition, and produced 2,2-
methylene-bis-6-methoxy-4-(2-propenyl)phenol
with yield of 43%. The other study also performed a
synthesis of 6-butylaminomethyl and 6-
dibutylaminomethyl from eugenol through Mannich
reaction with yield of 50 and 78%, respectively. This
study also learn the influence of the effect of
primary and secondary amines (Hecht, 2014).
(Popovici et al., 1999) synthesized several Mannich
oxime base derivatives into quaternary ammonium
salts. For the example, 1-(2-hydroxy-5-methylpenyl)
-3-dialkylamino-1-propanone was quaternized using
methyl iodide in tetrahydrofuran (THF) and ethanol
at room temperature with yield of 93 and 65%,
respectively. (Perangin-angin, 2019) synthesized 4-
allyl-6-(hydroxymethyl)-2-methoxyphenol from
eugenol through Mannich reactions followed by
methylation and substitution reaction.
Therefore, the objective of the present research
was to synthesize quaternary ammonium salt that
based on eugenol structure through Mannich
reaction and followed by methylation reaction. The
obtained quaternary ammonium salt showed a
moderate antibacterial activity.
2 MATERIALS AND METHODS
2.1 Materials
Equipment: glassware, rotary evaporator, hotplate
with stirrer, Fourier Transform Infrared (FT-IR),
Gas Chromatography Mass Spectrometer (GC-MS),
Spectrophotometer UV-Vis.
Materials: Eugenol, Dimethylamine, Ethanol,
Formaldehyde, Methyl iodide, Na
2
SO
4
anhydride,
Diethyl ether, Nutrient agar (NA), S. aureus, E. coli.
2.2 Synthesis of 4-Allyl-6-(Dimethyl
amino) Methyl-2-Methoxy Phenol
Compound
As much as 4.8 g of eugenol was dissolved with 28
mL of ethanol into the three neck round bottom flask
and then 3.8 g (3.48 mL ; 0.04 mol) of formaldehyde
(37 wt.%) and 5.6 g (6.3 mL ; 0.05 mol) of
dimethylamine (40 wt.%) were followed by reflux
process at 78
o
C for 90 minutes. The mixture was
cooled and stirred for 24 h. The excess ethanol was
then evaporated by rotary evaporator. The obtained
result was characterized by FT-IR and GC-MS.
2.3 Synthesis of 6-[(N-Iodo-N-Methyl-N
Methyl-N-Methylamino) Methyl] 4-
Allyl-2-Methoxy Phenol
As much as 4.404 g (0.2 mol) of 4-allyl-6-(dimethyl
amino)methyl-2-methoxyphenol was added into
erlenmeyer. Ethanol (35 mL) and methyl iodide (0.2
mol) were added into erlenmeyer, and tightly closed.
The mixture was stirred at room temperature with a
magnetic stirrer for 2 h and allowed to stand in the
refrigerator for one night. The precipitate formed
was filtered using filter paper and washed with
diethyl ether. The obtained product was purified by
recrystallization process using ethanol and analysed
by FT-IR.
2.4 Preparation of Nutrient Agar Slant
(NA)
About 7 g of NA was dissolved with 250 mL of
distillate water and sterilized in an autoclave at
121
o
C for 15 minutes.
2.5 Preparation of Medium Agar Slant
and Bacterial Culture Stock
The NA slant was prepared by adding 3 mL of NA
into test tube and placed it in the rack. Tilt the rack
onto solid surface so that the medium is slanted.
Allow the medium to harden in this position. The
culture was obtained from stock and taken with an
osse. This culture was incubated at 35
o
C for 18-24 h.
ICOCSTI 2019 - International Conference on Chemical Science and Technology Innovation
224
2.6 Preparation of Mueller Hinton
Agar (MHA) Medium
MHA (19 g) was entered into erlenmeyer and
dissolved with 500 mL of distillate water sterilized
in an autoclave at 121
o
C for 15 minutes.
2.7 Preparation of Bacterial Inoculum
Nutrient broth (3.25 g) was dissolved with 250 mL
of distillate water and sterilized in an autoclave at
121
o
C for 15 minutes. Furthermore, microbial
colony was taken from culture stock using a
sterilized osse. The culture was suspended into 10
mL of sterilized nutrient broth in the test tube and
incubated at 35
o
C for 3 h. The optical density of
bacterial was determined using spectrophotometer
UV-Vis at 580-600 nm.
2.8 Evaluation of Antibacterial Activity
The antibacterial activity of quaternary ammonium
salt was obtained by diffusion method. Paper disk (Ǿ
6 mm) had been soaked in various concentration of
quaternary ammonium salt (10, 20, and 30%). This
paper disk then placed on the agar medium that has
been cultured with E. coli and S. aureus. The
inhibition zone was measured using calliper (mm).
3 RESULTS AND DISCUSSION
3.1 Synthesis of 4-Allyl-6-(Dimethyl
amino) Methyl-2-Methoxy Phenol
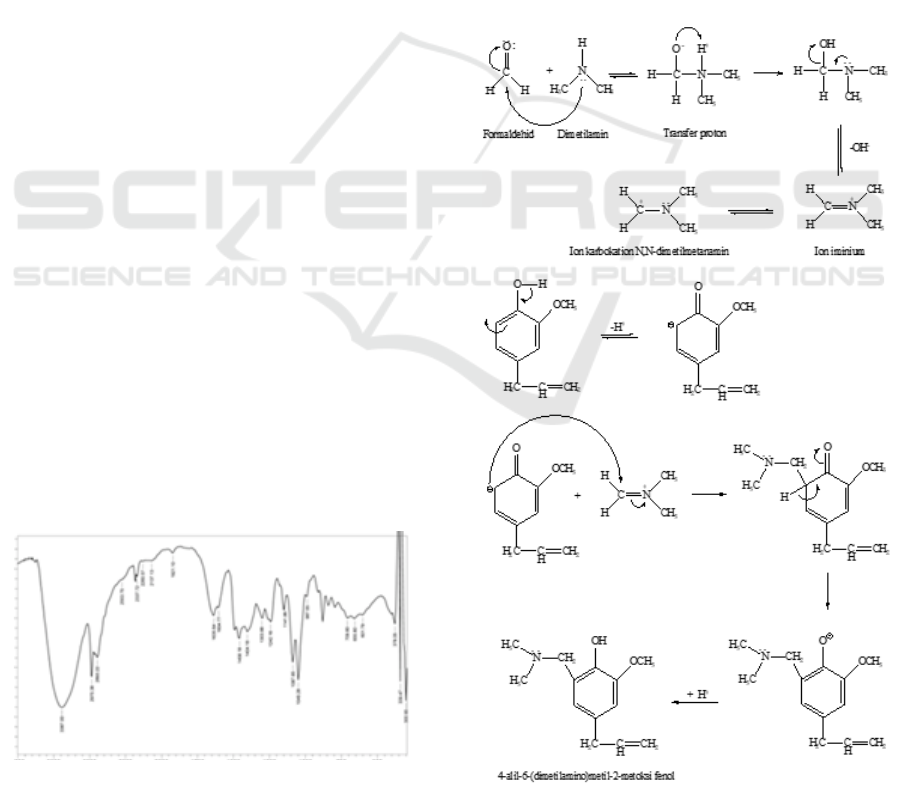
The ammonium quaternary salt, 4-Allyl-6-(dimethyl
amino)methyl-2-methoxyphenol, was obtained as
blackish brown liquid with amount of 5,51 g. The FT-
IR spectrum of 4-Allyl-6-(dimethyl amino)methyl-2-
methoxyphenol was showed in Figure 1.
Figure 1: FT-IR spectrum of 4-Allyl-6-(dimethyl
amino)methyl-2-methoxyphenol.
The spectrum showed that C-N bonding from
dimethylaminomethyl was exhibited at 1246.16 cm
-
1
. The exhibited signal at 3387 cm
-1
was identified as
vibration of OH stretching. The signal at 2970.38
and 2893.22 cm
-1
showed the vibration of aliphatic
CH, this signal was supported by the presence of
methylene signal at 1458.18 cm
-1
and methyl group
at 1303.88 cm
-1
. The presence of C=C aromatic was
shown at 1635.64 cm
-1
. The vinyl group was shown
at 987.55 cm
-1
and C-O-C from ether was shown at
1141.86 cm
-1
.
4-allyl-6-(dimethyl amino) methyl-2-methoxy
phenol was obtained from eugenol through the
Mannich reaction, which was reacted with iminium
ion. In the Mannich reaction of eugenol, the active
hydrogen from eugenol was replaced by the
dimethylaminomethyl. The mechanism reaction of
this quaternary ammonium salt formation was
displayed in Figure 2.
Figure 2: Mechanism reaction of the Mannich eugenol.
Synthesis of Quatenary Ammonium Compounds from Eugenol through Mannich and Methylation Reactions and Its Antibacterial Activity
225
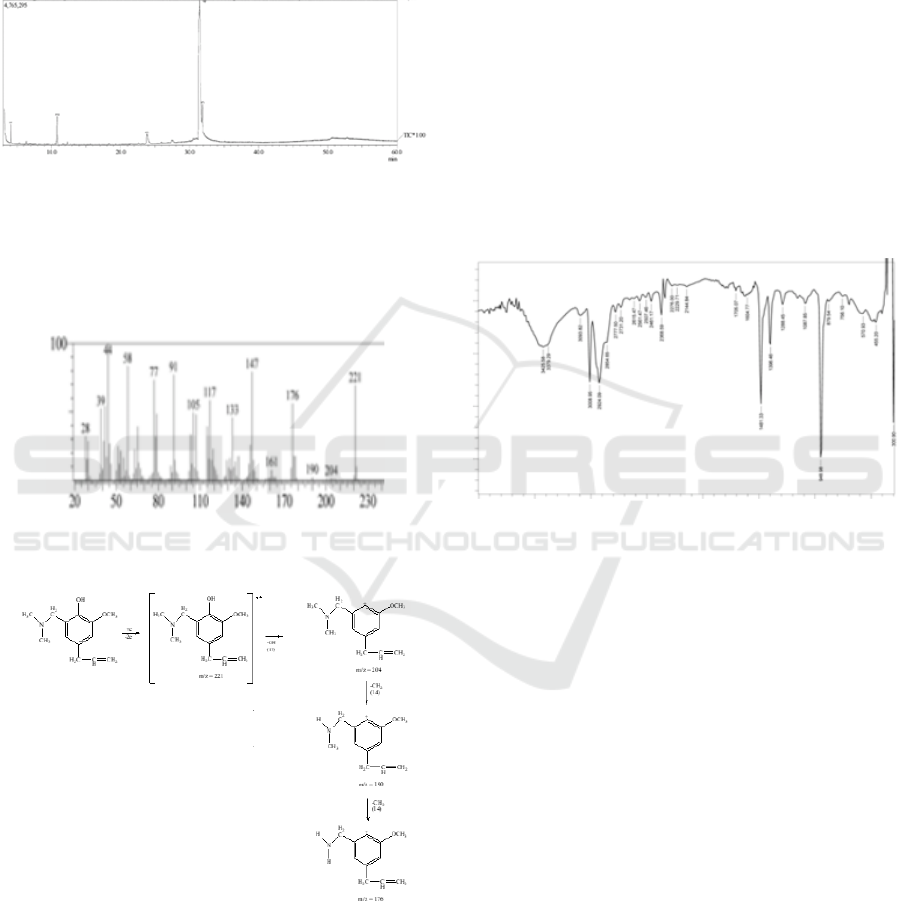
The obtained quaternary ammonium salt of 4-
allyl-6-(dimethyl amino) methyl-2-methoxyphenol
compound using GC-MS showed peak of retention
time at 31.428 minute with purity as much 85.65%.
The mass chromatogram of the compound
synthesized by GC-MS was showed in Figure 3.
Figure 3: FT-IR spectrum of 4-Allyl-6-(dimethyl
amino)methyl-2-methoxyphenol.
The obtained result of 4-Allyl-6-
(Dimethylamino)Methyl-2-Methoxy Phenol
spectrum was corresponded in Figure 4.
Figure 4: Mass spectrum of 4-allyl-6-(dimethylamino)
methyl-2-methoxy phenol compound.
Figure 5: Mass spectrum of 4-allyl-6-(dimethylamino)
methyl-2-methoxy phenol compound.
The peak of retention time for 31.428 minute
with molecular formula C
13
H
19
NO
2
that had relative
molecular mass was 221 g/mol. The Spectral data
showed molecular ion peaks at m/e 221 followed by
fragmentation peaks at m/e 204, 190, 176, 161, 147,
133, 117, 105, 91, 77, 58, 44, 39, and 28, which was
these values corresponded into the relative
molecular mass (Mr) of the 4-allyl-6-
(dimethylamino) methyl-2-methoxy phenol
compound synthesized. The fragmentation pattern
can be seen in Figure 5.
3.2 Synthesis of 6-[(N-Iodo-N-Methyl-
N-Methyl-N-Methylamino)
Methyl]-4-Allyl-2-Methoxy Phenol
Compound
In this study, the obtained result of 6-[(N-iodo-N-
methyl-N-methyl-N-methylamino) methyl]-4-allyl-
2-methoxy phenol was 5.71 in the form of yellow
solid and its FT-IR spectroscopy analyzed was
showed in Figure 6.
Figure 6: FT-IR spectrum of 6-[(N-iodo-N-methyl-N-
methyl-N-methylamino) methyl]-4-alyl-2-methoxy
phenol.
The FT-IR spectrum of 6-[(N-iodo-N-methyl-N-
methyl-N-methylamino) methyl)]-4-allyl-2-methoxy
phenol that can be found stretching vibration at
948.98 cm
-1
and supported by an absorption at
455,20 cm
-1
. Those Wavenumbers of 948.98 cm
-1
to
455.20 cm
-1
were typical of quaternary ammonium
salts that appeared simultaneously. The absorption
peak at 3379.29 cm
-1
was assigned as the O-H
vibration after that, the peak at 3008,95 cm
-1
was
corresponded as stretching vibration of C-H sp2
(=CH-) and the wavenumber at 2924,09 cm
-1
was
showed as C-H sp
3
on CH
2
. The absorption peak at
1481,33 cm
-1
indicated CH
2
and C=C aromatic was
showed at 1604.77 cm
-1
. The reaction of preparation
6-[(N-iodo-N-methyl-N-methyl-N-methylamino)
methyl]-4-allyl-2-methoxy phenol was showed in
Figure 7.
ICOCSTI 2019 - International Conference on Chemical Science and Technology Innovation
226
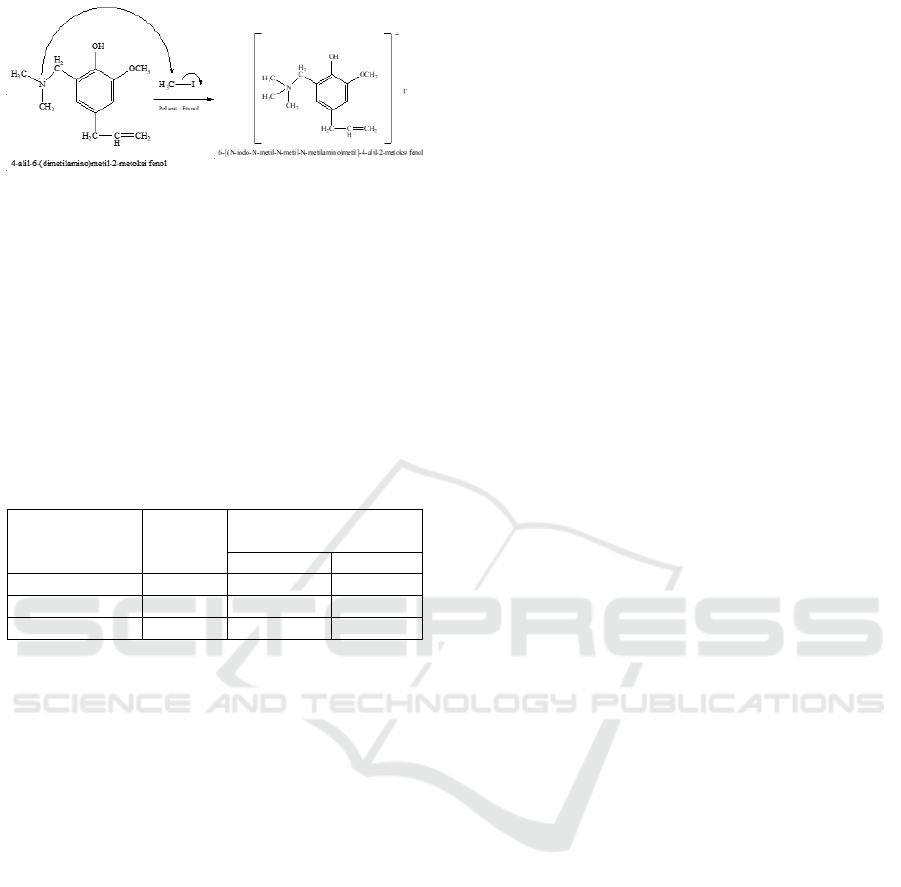
Figure 7: FT-IR spectrum of 6-[(N-iodo-N-methyl-N-
methyl-N-methylamino) methyl]-4-alyl-2-methoxy
phenol.
3.3 Antibacterial Activity
Antibacterial activity of 6-[(N-iodo-N-methyl-N-
methyl-N-methylamino) methyl]-4-alyl-2-methoxy
phenol using E. coli and S. aureus can be found in
Table 1.
Table 1: Antibacterial activity test of 6-[(N-iodo-N-
methyl-N-methyl-N-methylamino) methyl]-4-alyl-2-
methoxy phenol.
Treatment
Disc
diameter
(mm)
Clear zone diameter
(mm)
S. aureus
E. coli
10%
6
10.2
10.8
20%
6
10.8
11.4
30%
6
11.3
12.1
Based on antibacterial activity showed that 6-
[(N-iodo-N-methyl-N-methyl-N-methylamino)
methyl]-4-alyl-2-methoxy phenol had antibacterial
properties for both of S. aureus and E. coli. It was
caused that 6-[(N-iodo-N-methyl-N-methyl-N-
methylamino) methyl]-4-alyl-2-methoxy phenol had
a cationic charge amine group which was able to
bind food source of these bacteria that inhibited food
nutrition into bacterial cells (Nascimento et al.,
2000).
According to (Aleksandra et al., 2017) said that
antibacterial activity was classified to be 3 groups.
There were strong that produced inhibition zone
diameter at 8 mm, medium activity that produced
inhibition zone at 7-8 mm, while weak activity that
produced inhibition zone diameter less than 7 mm.
Therefore, quartenary ammonium compound has
strong antibacterial activity.
4 CONCLUSIONS
In this work, synthesis of 6-[(N-iodo-N-methyl-N-
methyl-N-methylamino) methyl]-4-allyl-2-methoxy
phenol was performed by 2 step, namely Mannich
and Methylation reactions. The obtained result of 4-
allyl-6-(dimethylamino) methyl-2-methoxy phenol
was 5.51 g with a yield of 83.03% and 6-[(N-iodo-N
-methyl-N-methyl-N-methylamino) methyl]-4-alyl-
2-methoxy phenol was 5.71 g with a yield of
78.39%. The characterization by FT-IR confirmed
the existence of 6-[(N-iodo-N-methyl-N-methyl-N-
methylamino) methyl]-4-allyl-2-methoxy phenol as
quaternary ammonium salt to the stretching
vibrations of C-N+ and the peak at 948.98 cm
-1
and
supported by absorption vibration C-N
+
at 455.20
cm
-1
. Furthermore, 6-[(N-Iodo-N-methyl-N-methyl-
N-methylamino) methyl]-4-allyl-2-methoxy phenol
can be used as antibacteria which showed
antibacterial activity for both of E.coli and S. aureus.
This compound showed antibacterial activity for E.
coli was better compared to S. aureus and classified
to strong antibacterial activity.
ACKNOWLEDGEMENTS
Author would like to thank to Rector of University
of Sumatera Urara for the funding from the project
of PD-TALENTA 2019
REFERENCES
Aleksandra, O. B., N, D. Z., Rzepkowska, A., Ko, D.,
2017. Comparison of Antibacterial Activity of
Lactobacillus plantarum Strains Isolated from Two
Different Kinds of Regional Cheeses from Poland :
Oscypek and Korycinski Cheese 2017.
Hecht, M., 2014. Synthesis and antimalarial activity of
new 5–10.
Karanov, E., Iliev, L., Alexieva, V., Georgiev, G. T.,
Thang, N. T., Natova, L., 1995. Synthesis and Plant
Growth Regulating Activity of Some Novel 2-
Methoxy-4-(1-or 2-Propenyl)-6-Substituted Phenols
21, 39–47.
Madigan, M. T., 2005. Brock Biology of Microorganisms,
15th ed. Prentice Hall.
Nascimento, G. G. F., Locatelli, J., Freitas, P. C., Silva, G.
L., 2000. Antibacterial activity of plant extracts and
phytochemicals on antibiotic-resistant bacteria.
Brazilian J. Microbiol. 31, 247–256.
Perangin-angin, S., 2002. Pembuatan Turunan Amina dari
Eugenol dan Reaksi Turunan Amina Basa Mannich.
Universitas Gajah Mada.
Perangin-angin, S., 2019. JCNaR Phenol Compounds from
Eugenol Through Mannich Reaction Followed
Methylation with Methyl Iodide and Substitution
Using NaOH 01, 75–85.
Popovici, I., Comanitǎ, E., Roman, G., Comanitǎ, B.,
1999. Synthesis and reactivity of some mannich bases.
Synthesis of Quatenary Ammonium Compounds from Eugenol through Mannich and Methylation Reactions and Its Antibacterial Activity
227

IV. Quaternary ammonium iodides from mannich
bases oximes. Acta Chim. Slov. 46, 413–420.
Purwono, B., Daruningsih, E. R. P., 2010. Nucleophilic
Substitution Reaction of Cyanide and Methoxyde Ions
to Quaternary Mannich Base from Vanillin. Indones.
J. Chem. 5, 203–206.
Sastrohamidjojo, H., 2004. Kimia Minyak Atsiri. Gadjah
Mada University Press, Yogyakarta.
Stanley, P. H., 2011. pine2011.pdf 403–464.
Towaha, J., 2012. The benefits of cloves eugenol in
various industries in Indonesia. J. Perspekt. 11, 79–90.
ICOCSTI 2019 - International Conference on Chemical Science and Technology Innovation
228
