
Preparation and Characterization of Tungsten Trioxide (WO
3
)
Particles and Their Photocatalytic Performances for Methylene Blue
Degradation
Lusi Ernawati
1
, Ruri Agung Wahyuono
2
, Inggit Kresna Maharsih
1
, Ade Wahyu Yusariarta
1
1Institut Teknologi Kalimantan
2
Institut Teknologi Sepuluh Nopember
Keywords: Photodegradation, organic dye, methylene blue, photocatalytic, UV irradiation, WO
3
Abstract: Tungsten trioxide (WO
3
) nanoparticles were successfully synthesized using sodium tungstate dihydrate
(Na
2
WO
4
.2H
2
O, as WO
3
source) via facile sol-gel method. This study aims to synthesize WO
3
and to
investigate the effects of Na
2
WO
4
precursor concentration on the particle morphology, crystallinity, and
photocatalytic performance. The prepared particle can be activated under UV irradiation and showed good
photocatalytic efficiency for methylene blue (MB) degradation. The results showed WO
3
dose-dependent
photocatalytic performance toward 10 mg/L MB degradation. The adsorption kinetics of MB to the WO
3
catalyst surface can be evaluated and fit by using the pseudo-first-order kinetic adsorption model. The
photodegradation test showed that the concentration (C
t
/C
o
) of 180 ml of MB decreases rapidly up to 88%
with 110 mg of WO
3
for 2h irradiation.
1 INTRODUCTION
There are more than 700,000 tons of different dyes
substances annually produced, among which 15%
are discharged as effluent into the environment by
industries such as textiles, rubber, leather, plastics,
and food (Him et al., 2019). It is well known that
wastewater treatment, particularly in the textile and
dye industry, mainly involves the treatment of
highly colored wastewater containing a variety of
dyes in different concentrations. This dye-
contaminated wastewater can cause harmful damage
to the ecosystem and health, for example, increasing
the DO (Dissolved Oxygen) level in the polluted
ecosystem, which will result in an increase in COD
(Chemical Oxygen Demand) (Chong et al., 2010;
Coleman et al., 2007; Dai et al., 1999; Ernest et al.,
2010).
There are a variety of adsorbents developed for
dye removals that have been studied, among which
activated carbons are most widely used. However,
their current applications are limited due to their
relatively high cost (Fujishima et al., 2001). Other
alternative technologies are also developed for the
decoloring process, for example, by coagulation
techniques, flocculation, adsorption with activated
carbon (Halliday et al., 2011). Nonetheless, it has
been found that the color removal using these
technologies only transforms the dyes from the
liquid into the solid phase while they do not degrade
the dye into less harmful compounds (Indonesia’s
Garment and Textile Sector, 2018).
Considering the limitation of the
abovementioned treatment process, studies of
photocatalytic degradation of organic
dyes/pollutants are growing. Amongst various
photocatalyst, titanium oxide (TiO
2
) is considered
the suitable materials for photodegradation of
organic compounds as it is inexpensive, largely
available, thermally stable, and harmless [9].
However, this material has a relatively wide energy
bandgap (Eg) of 3.2 eV, which limits further
applications of the material in the visible-light
region (λ + 390 nm) (Ke et al., 2018). In this regard,
tungsten trioxide (WO
3
) has been proposed as an
attractive candidate due to its high stability in
aqueous solution under acidic conditions.
Furthermore, its low energy bandgap (Eg) of 2.4-2.8
eV (Kang et al., 2001) allows for a photocatalytic
process triggered under visible solar spectrum (Kim
et al., 2006) and the conduction band level of WO
3
is suitable to allow favorable charge transfer to
Ernawati, L., Wahyuono, R., Maharsih, I. and Yusariarta, A.
Preparation and Characterization of Tungsten Trioxide (WO3) Particles and Their Photocatalytic Performances for Methylene Blue Degradation.
DOI: 10.5220/0009193000050011
In Proceedings of the 1st International Conference on Industrial Technology (ICONIT 2019), pages 5-11
ISBN: 978-989-758-434-3
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
5

generate intermediates OH• responsible for pollutant
degradation (Michalow et al., 2009).
While the preparation of WO
3
nanoparticles is
often reported using complicated synthetic routes
that require high-temperature process, pressure, and
expensive apparatus (Kang et al., 2001; Kim et al.,
2006; Michalow et al., 2009), the work at hand
presents a facile way and low-cost approach to
synthesize WO
3
nanoparticle via sol-gel method at
mild temperature. The crystallite morphology and
structure are discussed upon varying the ratio
between precursor and surfactant. The dose-
dependent of WO
3,
as well as the dye concentration-
dependent to photocatalytic activities for the
Methylene Blue (MB) degradation, are studied in
detail.
2 EXPERIMENTAL METHODS
2.1 Synthesis of WO
3
Particles
The synthesis was conducted through acidic
precipitation method using Na
2
WO
4
.2H
2
O under
mild condition. An amount of Na
2
WO
4
.2H
2
O was
dissolved in 50 ml of aqua dest and stirred for 30
min. Subsequently, 2 M of HCl was added into the
solution to reach pH < 6 and heated up to 90
o
C.
Afterward, the CTABr solution was poured into the
mixture (with 1 :1, 2 :1, and 4 :1 ratio to
Na
2
WO
4
.2H
2
O), and white precipitation was formed.
The mixture solution was kept at 90
o
C and stirred
for another 30 min. Finally, the precipitates were
filtered, dried in an oven (100
o
C, 1 h), and annealed
in the furnace at 500
o
C for 4 h.
2.2 Photocatalytic Performance of
WO
3
Particle for Methylene Blue
Degradation
Photocatalytic activity of WO
3
against organic
pollutants, i.e., methylene blue (MB), was assessed.
MB photodegradation tests were carried out
employing a different dose of WO
3
catalysts and
different concentrations of MB. The photocatalyst
was soaked in the aqueous MB solution and then
transferred into a custom-built photoreactor and
irradiated under UV light (T5-UV7-W, 254 nm in
wavelength) for several times, i.e., every 15 min for
2 h. The solution was stirred to increase contact
between photocatalyst and MB molecules. In
addition, the reactor was isolated from the ambient
light irradiation. Photodegradation of MB was
detected through absorption change at 665 nm
measured using a UV/vis spectrometer (Rayleigh
UV-9200). The decrease of MB optical density was
used to determine the decreasing MB concentration
due to the catalytic activity of the WO
3
catalyst.
2.3 Characterization
X-Ray Diffraction (XRD) patterns were obtained
using a PAN analytical type X’Pert Pro
diffractometer with Cu-Kα as the radiation source
operated at 40 kV and 40 mA. Samples were
scanned between 10 and 100° diffraction angle (2θ)
with a resolution of 0.05°. Crystallite size was
estimated using the Debye-Scherrer equation. SEM
image of WO3 powder was measured by scanning
electron microscopy (SEM, FEI type Inspect 21) at
100 kV accelerating voltage. Fourier-transform
infrared (FTIR) spectroscopy was carried out using
PerkinElmer Spectrum version 10.5.1.
3 RESULTS AND DISCUSSIONS
3.1 Physical and Microstructural
Properties of WO3 Particle
SEM micrographs (Figure 1) showed the
morphology of the resultant WO
3
particles by the
different concentration of sodium tungstate,
including 0.1; 0.2 and 0.3 M after annealing at
500
o
C for 4 h. The morphology of synthesized WO
3
particles exhibits a small spherical shape.
Nonetheless, WO
3
particles show particle size
distribution with a high polydispersity for all molar
ratio used in this work, Na
2
WO
4
(0.1 M, Dp=
42.16) ; Na
2
WO
4
(0.2 M, Dp= 55.27) ; and Na
2
WO
4
(0.3 M, Dp= 47.72). Hence, the results indicate that
the concentrations of Na2WO4 play an insignificant
rôle to tune the particle size. In addition, aggregation
is observed as a small WO
3
particle tends to be
unstable and easier to form aggregates than larger
particles (Szekely et al., 2016). The aggregation is
also plausibly formed due to interactions and
collisions between particles (Morales, 2008).
ICONIT 2019 - International Conference on Industrial Technology
6
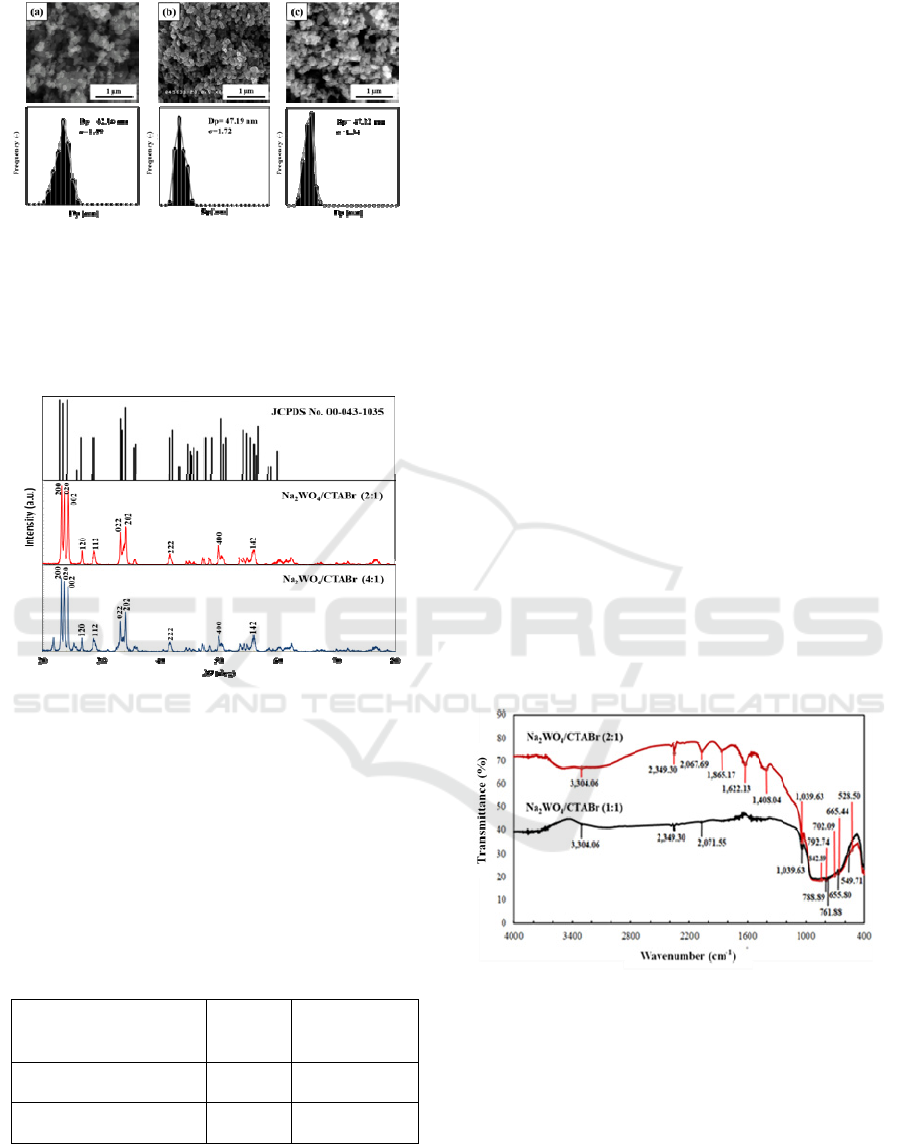
Figure 1. Morphology of synthesized WO3 particle with
variation of Na2WO4 concentration (a) 0.1 M, (b) 0.2 M
and (c) 0.3 M.
The microstructures of prepared WO
3
particles
were characterized by XRD analysis. The results are
shown in Figure 2.
Figure 2. XRD powder diffractogram of WO
3
with
variations composition of Na
2
WO
4
/ CTABr.
According to JCPDS (No. 00-043-1035), the
prepared WO3 shows characteristic of a monoclinic
WO3 crystal structure indicated by diffraction angle
2ϴ at 23.15°, 23.66°, 24.37°, 26.63°, 28.76°, 33.30°,
34.16°, 41.71°, 49.96°, and 55.93°. The crystallite
size is then estimated using the Scherrer equation
(Table 1).
Table 1. The crystallinity of WO
3
particles with different
composition of Na2WO4/CTABr
WO
3
Sample
Crystal
Size
(nm)
Crystallinity
(%)
Na
2
WO
4
/CTABr (2:1)
40,39 89,33
Na
2
WO
4
/CTABr (4:1)
45.68 90,01
The crystallinity of synthesized WO
3
particle, as
shown in Table 1 also does not show a significant
difference, i.e., the crystallinity is found as high as
90%. It should be noted that the crystallinity is
influenced by the calcination temperature. The
higher the calcination temperature of WO
3
, the
higher the crystallinity (Palupi, 2006). The
crystallite size of WO
3
using Na
2
WO
4
/CTABr (4:1)
is larger than WO
3
Na
2
WO
4
/CTABr (2:1). It is
indicating that CTABr, as a surfactant, is able to
prevent collisions among WO
3
particles during
nucleation leading to a slower formation of WO
3
particles as well as the nucleation rate. Therefore,
the size of the WO
3
particles is getting smaller with
respect to the higher concentration of CTABr (Papp,
1994).
3.2 FTIR Spectra of WO3 Particle
The functional groups of WO
3
particles were
characterized by FTIR, as shown in Figure 3. The IR
spectra exhibit distinct peaks between 400 and 4000
cm
-1
. Both WO3 particles show a peak at 3304.06
cm
-1
, which assigned to the vibration of O-H bonds
of water content in WO
3
(Patel and Vashi, 2015).
Notably, WO
3
particle with the composition of
Na2WO4/CTABr (2:1) shows the OH group
vibration at 1622.13 cm-1 and 1408.04 cm-1
(Petsom et al., 2018). In addition, both WO3
particles exhibit IR peaks at 1039.63 cm-1 that can
be ascribed to W-OH bond vibration (Sanchez-
Martinez et al., 2014). Further IR fingerprint of W-O
bonds is characterized by peaks within the range of
500-1000 cm
-1
.
Figure 3. FTIR spectra of WO
3
Compounds with the
varied composition of Na
2
WO
4
/ CTABr.
Comparing these two particles, WO
3
prepared
using Na
2
WO
4
/ CTABr (2:1) shows a strong vibration
at 842.89, 792.74, 702.09, 665.44, and 528.50 cm
-1
.
Meanwhile, WO
3
prepared using Na
2
WO
4
/ CTABr
(1:1) exhibit slightly different strong vibration bands
at 788.89, 761.88, 655.80, and 549.71. All of these
bands can be indicative of a W-O-W bond (Szekely
et al., 2016). Thus, it can be deduced that pristine
WO
3
particles (without impurities) are obtained.
Preparation and Characterization of Tungsten Trioxide (WO3) Particles and Their Photocatalytic Performances for Methylene Blue
Degradation
7
3.3 Effect of WO
3
amount on MB
Degradation
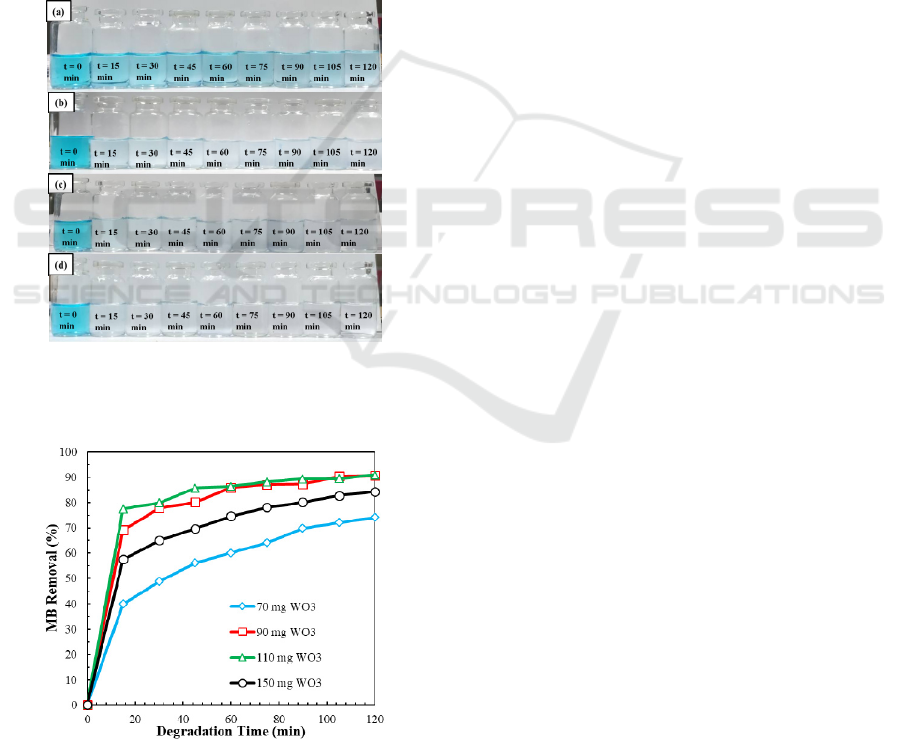
The initial investigation of photocatalytic activity is
carried out to understand the effect of the WO
3
dose
on the photocatalytic degradation of MB as organic
pollutants. The photoreactor containing 10 ppm of
MB solution and the amount of WO
3
particles tested
is varied by 70 mg, 90 mg, 110 mg, and 150 mg.
The photographs of MB degradation with variations
amount of WO
3
is depicted in Figure 4. Upon the
pictorial view, 70 mg of WO
3
cannot fully degrade
the MB after 120 minutes’ irradiation. However, the
colorless solution is observed upon using at least 90
mg WO
3
catalyst after 120 minutes irradiation. In
addition, maximum degradation is obtained using
150 mg of WO
3
.
Figure 4. Photographes of MB degradation at 120 minutes
using variation amount of WO
3
particle (a) 70, (b) 90, (c)
110, and (d) 150 mg.
Figure 5 The time-dependent MB concentration using
different amounts of WO
3
particle. The amount of MB (10
ppm) was kept constant.
The quantitative results of MB degradation by
WO
3
particle are shown in Figure 5. It is shown that
at least 70% of MB is degraded using 70 mg of the
catalyst after irradiation. The photocatalytic activity
tends to be low at a minimum dose of 70 mg
(39.89% degradation) at 15 minutes, while the high
and rapid photocatalytic activity is obtained using
150 mg, i.e., 95.8% MB is degraded within 15
minutes. Overall, the results show that the rate of
MB degradation depends on the dose of the WO
3
catalyst. The higher concentration of catalyst used
will increase MB percentage removal because the
surface area of the catalyst increases. The increased
surface area influences the number of existing active
sites and the magnitude of the reaction rate of the
photo-generated electron-hole pairs on the surface to
react with water creating more oxidant agents, which
increases the efficiency of photocatalytic activity
(Wang et al., 2019).
It is interesting to note that 110 mg and 150 mg
dose of catalyst do not show significant difference
since the available active sites provided using 110
mg are already sufficient (reach a saturated value) to
adsorb the amount of MB in solution. The stagnant
and even decreasing degradation upon increasing the
dose of catalyst can also be caused by increasing
turbidity. Increasing the turbidity of the solution
decreases the absorption of light, which related by
the number of particles capable of capturing photons
and producing reactive oxidants to degrade organic
compounds (Werth et al., 2003).
3.4 Effect of MB Concentration
Further investigation is carried out to understand the
effect of MB concentration on the photocatalytic
degradation process. In this regard, the photoreactor
containing 10 mg mL-1 of WO
3
particles was used,
and the concentration of MB solution tested was
varied by 5, 10, 15, and 20 mg L-1. The qualitative
results are indicated in Figure 6, while the
quantitative results are shown in Figure 7.
It is apparent that the concentration of MB as a
coloring agent affects the photocatalytic activity
(Figure 7). The concentration of MB at 5 mg L
-1
has
the largest MB removal, which reaches 100% while
at 10 mg L
-1
MB is the smallest MB removal
(88.7%). For 20 mg L
-1
concentration is MB
removal marked by 90%, while 94% MB removal is
obtained for 15 mg L
-1
concentration. The results
obtained above can be explained as follow: The less
concentrated MB solution (5 mg L
-1
) can increase
the degradation rate because the oxidants OH•
produced by the reaction between catalysts and
ICONIT 2019 - International Conference on Industrial Technology
8
specific H
2
O and H
2
O
2
are able to degrade all MB in
75 minutes. However, in this study, the smallest %
MB removal is owned by the MB concentration of
10 mg L
-1
, which can be due to randomly limited
MB adsorption on the surface of the catalyst and
diffusion at the active site of the catalyst (Wicaksana
et al., 2014). When the MB concentration is
sufficiently high, more MB can be adsorbed and
diffused at the active site of the catalyst. As a result,
more OH• are generated and able to efficiently
degrade MB on the surface catalyst (Zheng et al.,
2011).
Figure 6. Photographes of MB degradation after 120
minutes’ irradiation using variation concentration of MB
(a)5 mg/L, (b) 10 mg/L, (c) 15 mg/L, (d) 20 mg/L.
Figure 7 The corresponding percentage of MB degradation
under various MB concentration. The amount of
WO
3
(110 mg) and stirred at 300 rpm.
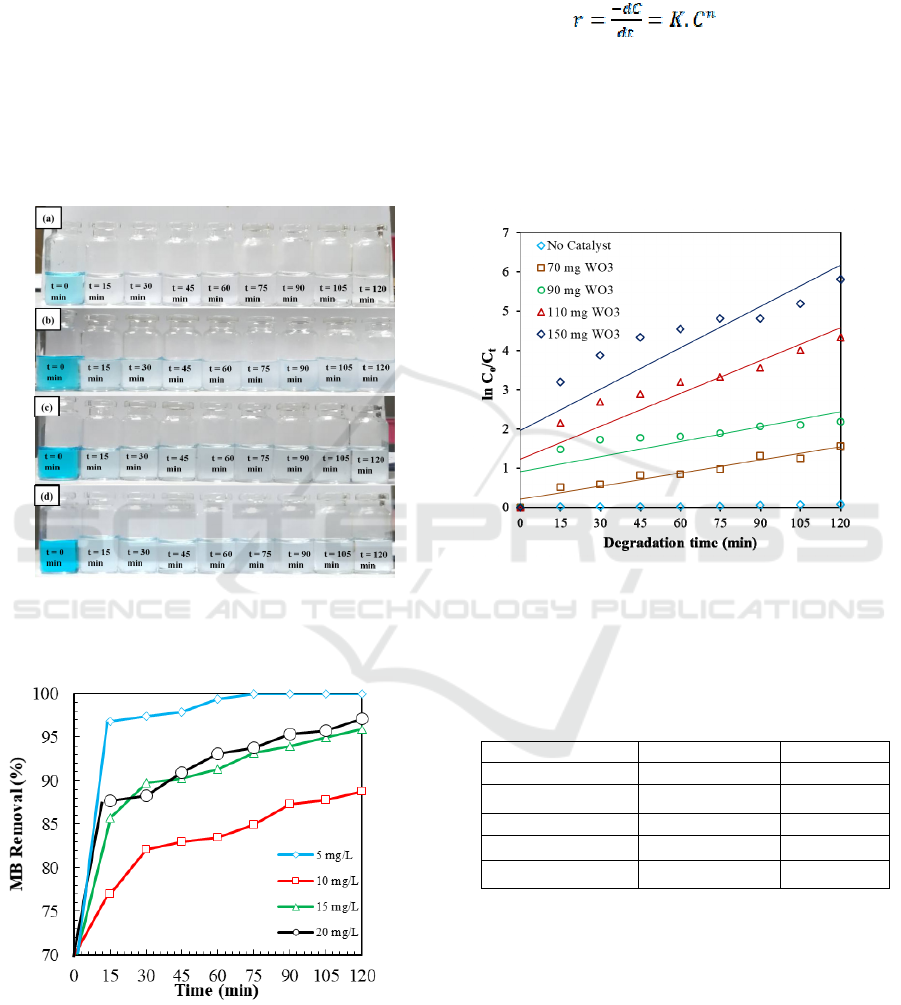
In this study, the reaction rate constant (K) is
approached and determined using the homogeneous
system following first-order reaction (n =1). Kinetics
of photodegradation using different amount of WO
3
catalyst is therefore evaluated following pseudo-
first-order kinetic expression:
(3)
Where K is the reaction rate constant, and n is
the order of the reaction. The order of reaction and
reaction rate constants are determined from the
integration of the reaction rate equation resulting in
a linear equation, in which K is the slope of the
linear curve.
Figure 8. Time-dependent ln (Co/Ct) for photodegradation
of MB (10 mg L
-1
) under different amount of WO
3
particles (70, 90, 100, and 150 mg).
Table 2. Reaction rate constants (K) and the coefficient of
determination (R
2
) obtained for photodegradation of MB
using different amount of WO
3
WO
3
amount k ( min
-1
) R
2
No Catalyst k1 = 0.0006 0.9073
70 mg k2 = 0.0111 0.9383
90 mg k3 = 0.0127 0.6198
110 mg k4 = 0.0279 0.8097
150 mg k5 = 0.0351 0.7227
The kinetic study reveals that pseudo-first-order
reaction is sufficient to fit the kinetic data. Hence, it
can be understood that the MB molecules are
physisorbed on the catalyst surface prior to exposing
the photocatalytic process. The kinetic study also
indicates that the increasing amount of WO
3
induces
the rate of reaction to increase as indicated by the
increasing slope at a higher amount of WO
3
. It is
apparent that the amount of WO
3
influences the rate
of the reaction (Table 2). The catalyst amount of 150
mg has the highest reaction rate (0.0351 min
-1
),
Preparation and Characterization of Tungsten Trioxide (WO3) Particles and Their Photocatalytic Performances for Methylene Blue
Degradation
9

while the catalyst amount of 90 mg has the lowest
reaction rate (0.0111 min
-1
). The results obtained
here show promising performance as compared to
the reaction rate obtained for other WO
3
nanostructures reported in the literature (Zheng et
al., 2011; Ernawati et al., 2019).
4 CONCLUSIONS
WO
3
nanoparticles were successfully synthesized by
acidic precipitation-assisted sol-gel method using
Na
2
WO
4
.2H
2
O as a precursor and CTABr as a
reactive agent. It is found that the composition of
CTABr and Na
2
WO
4
.2H
2
O during the synthesis
affects the aggregation formation of WO
3
nanoparticles. However, varying composition does
not yield a significant difference in the crystallinity
of nanoparticles. The photocatalytic degradation test
of MB in aqueous medium indicates a WO
3
dose-
dependent performance as well as MB
concentration-dependent performance. The kinetic
study unravels that the initial mechanism of MB
degradation using WO
3
is physisorbtion of the dye
molecules on catalyst surface as indicated by the
pseudo first-order kinetic fit. The highest reaction
rate constants were obtained by using 150 mg of
WO
3
catalyst (k5 = 0.0351 min
-1
).
ACKNOWLEDGMENT
The author would like to thank Laboratorium Pusat
Sentral Material Maju dan Terbarukan (Universitas
Negeri Malang) for technical assistant of material
characterizations. This research is supported by
Lembaga Penelitian dan Pengabdian Masyarakat
(LPPM) Institut Teknologi Kalimantan, Indonesia.
REFERENCES
Chi Him, A. T., Kai, L., Yuxuan, Z., Wei, Z., Tao, Z.,
Yujie, Z., Ruijie, X., Dennis, Y. C. L., Haibao, H.
2019. Titanium oxide Based photocatalytric materials
development and their role for the air pollutant
degradation: overview and forecast. 125, 200-228.
Chong, M. N., Jin, B, Chow, C. W, Saint, C., 2010. Recent
developments in photocatalytic water treatment
technology: A review. Water Res 44:2997–3027.
Coleman, H. M., Vimonses, V., Leslie, G., Amal, R.,
2007. Degradation of 1,4-dioxane in water using TiO
2
based photocatalytic and H
2
O
2
/UV processes. J.
Hazard Material, 146, 496-501.
Dai, Q., Zhang, Z. He, N., Li, P. Yuan, C., 1999.
Preparation and Characterization of Mesostructured
Titanium Dioxide and Its Application as a
Photocatalyst for the Wastewater Treatment. J.
Materials Science and Enginering. 8-9, 417-423.
Ernest M.H., Tanapon P., Gregory V. L., 2010.
Nanoparticle Aggregation: Challenges to
Understanding Transport and Reactivity in the
Environment. J. Quality, 39, 1909-1924, Carnegie
Mellon University, Qatar.
Fujishima, A., Rao, T. N., Tryk, D. A., 2001. Titanium
dioxide Photocatalysis. J. Photocem and Photobio, 1,
1- 21.
Halliday, D., Resnick, R., Walker, J., 2011. Fundamentals
of Physics. Hoboken, N. J. Wiley.
Indonesia’s Garment and Textile Sector, 2018. Remain
Optimistic Amid Mounting Pressure. Global Business
Guide Indonesia.
J. J. Moses., L. Ammayappan., 2015. Growth of textile
industry and their issues on environment with
reference to wool industry.
Ke, D., Liu, H., Peng, T., Liu, X., 2008. Preparation and
photocatalytic activityof WO
3
/TiO
2
nanocomposite
particles. J. Materials Letters, 62, 447-450.
Kang, Y. S., Myun, K. P., Young, T. K., Hyun, W. L.,
Won, J. C., Wan, I. L., 2001. Preparation of
Transparent Particulate MoO
3
/TiO
2
and WO
3
/TiO
2
Films and Their Photocatalytic Properties. J. Catalyst.
191, 192-199.
Kim, J. O., Traore, M. K., Warfield, C., 2006. The textile
and apparel Industry in Developing Countries. Textile
Progress, 38(3), 1-64.
Michalow, K. A., Heel, A., Vital, A., Amberg, M.,
Fortunato, G., Kowalski, K., Graule, T.J., Rekas, M.,
2009. Effect of Thermal Treatment on the
Photocatalytic activity in Visible Light of TiO
2
-W
flame Spray Synthesized Nanopowders., Top. Catal.
52, 1051-1059.
Morales, W., 2008. Combustion Synthesis and
Characterization of Nanocrystalline WO
3
. The
University of Texas at Arlington, Arlington.
Palupi, E., 2006. Degradasi Methylene Blue dengan
Metode Fotokatalisis dan fotoelektrokalasis
menggunakan film TiO
2
. Skripsi. Institut Teknologi
Bandung.
Papp, J., Soled, S., Dwight, K., Wold, A., 1994. Surface
Acidity and Photocatalytic Activity of TiO
2
,
WO
3
/TiO
2
and MoO
3
/TiO
2
Photocatalysts. Chem.
Mater. 6, 496-500.
Patel, H., Vashi, R. T., 2015. Characterization and
treatment of textile wastewater. Elsevier: 3 -5.
Petsom, K., Kopwitthaya, A., Horphathum, M.,
Ruangtaweep, Y., Sangwarantee, N., Kaewkhao, J.,
2018. Shape-controlled synthesis of tungsten oxide
nanostructures and characterization. J. Metals,
Materials and Minerals, 28, 69-75.
Sanchez-Martinez, D., Hernandez-Uresti, D. B., Cruz, A.
M. L., Guzman-Sepulveda, S., Torrez-Martinez, L.
M., 2014. Characterization and Photocatalytic
properties of hexagonal and monoclinic WO
3
prepared
ICONIT 2019 - International Conference on Industrial Technology
10

via microwave-assisted hydrothermal synthesis. J.
Ceramics, 40:4767-4775.
Szekely, I., Kovacs, G., Baja, L., Danciu, V., Pap. Z.,
2016. Synthesis of shape-tailored WO
3
micro/nanocrystals and the photocatalytic activity of
WO
3/
TiO
2
composites. J. Materials, 9(258), 1-14.
Wang, W. W., Fu, H. T., Yang, X. H., An, X. Z., 2019.
Preparation and visible-light-driven photocatalytic
activity of WO
3
/TiO
2
core-shell nanorods,
International Workshop on Materials Science and
Mechanical Engineering, 504.
Werth J.H., M. Linsenbuhler, S.M. Dammer, Z. Farkas, H.
Hinrichsen, K.-E Wirth, dan D.E. Wolf., 2003.
Agglomeration of Charged in Nanopowder
Suspensions, Germany.
Wicaksana, Y., Liu, S., Scott, J., Amal, R., 2014. Tungsten
Trioxide as a Visible Light Photocatalyst for Volatice
Organic Carbon Removal. J. Molecules, 19, 17747-
17762.
Zheng, H., Ou, J.Z., Strano, M.S., Kaner, R.B., Mitchell,
A., Kalanta-zadeh, K., 2011. Nanostructured Tungsten
Oxide-Properties, Synthesis, and Applications.
Adv.Funct. Mater. 21(12), 2175–2196.
Ernawati, L., Wahyuono, R. A., Muhammad, A. A.,
Nurislam Sutanto, A. R., Maharsih, I. K., Widiastuti,
N., Widiyandari, H., 2019. Mesoporous WO
3
/TiO
2
Nanocomposites Photocatalyst for Rapid Degradation
of Methylene Blue in Aqueous Medium. International
Journal of Engineering TRANSACTION A: Basics, 32,
1345-1352.
Preparation and Characterization of Tungsten Trioxide (WO3) Particles and Their Photocatalytic Performances for Methylene Blue
Degradation
11
