
Sustainability of Financing to Increase Drug Access and Distribution
during National Health Insurance (JKN)
Andi Leny Susyanty
1
, Yuyun Yuniar
1
and Selma A. Siahaan
2
1
Centre for Health Resources and Services Research and Development, National Institute of Health and Development,
Ministry of Health, Jl. Percetakan Negara No. 29, Jakarta 10560, Indonesia
2
Centre for Health Humaniora and Health Management Research and Development, National Institute of Health and
Development, Ministry of Health, Jl. Percetakan Negara No. 29, Jakarta 10560, Indonesia
Keywords: Drug, Medicine, Cost, Distribution, Funding.
Abstract: Drug access for the public is largely influenced by four main factors, namely rational drug use, affordable
prices, sustainable funding, and a health system and a reliable drug supply system. The study carried out
qualitative and quantitative mixed methods with cross-sectional designs. Data collection is done through in-
depth interviews and secondary data collection. The study was conducted in February-December 2017. The
research sites in 11 provinces were selected purposively and divided into five regions were in accordance
with the Indonesian Case-Based Groups (INA-CBGs) System. The results of the study show that the costs
of purchasing drugs are sourced from the APBN and APBD, as well as capitation in several regions. For
medicine, there is generally no problem with the cost of drug distribution. The problem of additional
distribution costs occurs for the supply of medical devices. The anticipation of drug vacancies due to a
shortage of APBN and DAK funds can be overcome by optimizing JKN capitation funds by imitating
existing funding models. The supply of e-catalog drugs needed to be continuously evaluated by taking into
account the certainty of the prices and availability of goods.
1 INTRODUCTION
Since decentralization, there was a shift in the
management of Government funds, a significant
increase in Regional Government in line with
decentralization. Health financing comes from
various sources, namely: Central Government,
Regional Government, private sector, community
organizations, and the community itself. The
availability of adequate funding will also support the
implementation of subsystems for pharmaceutical
preparations, medical devices, and food (Presidential
Regulation, 2012).
One of the aimed pharmaceuticals, medical
devices, and food subsystems is to ensure the
availability, equity, and affordability of medicines,
especially essential medicines. Sufficient funding
from the Government and Local Governments is
needed to guarantee the availability and affordability
of drugs, especially drugs and essential medical
devices for the poor (Presidential Regulation, 2012).
Drug access for the public is largely influenced
by four main factors, namely rational use of drugs,
affordable prices, sustainable funding, and a reliable
health system and drug supply system. Availability,
equity, and affordability of drugs are achieved,
among others, through sustainable drug financing
system strategies, both the public sector and the
private sector (Ministry of Health, 2006).
One of the objectives from Distribution
Availability, And Drug and Vaccine Service In
Facing The 2019 Universal Health Coverage
Research study is to identify and assess the
distribution and availability of drugs and vaccines in
five regions of Indonesia where one of the specific
objectives is to calculate the financing components
of drug and vaccine distribution (Yuniar, 2017).
2 METHOD
The study carried out qualitative and quantitative
mixed methods with cross-sectional designs. Data
collection is done through in-depth interviews and
secondary data collection. The study was conducted
in February-December 2017.
Susyanty, A., Yuniar, Y. and Siahaan, S.
Sustainability of Financing to Increase Dr ug Access and Distribution during National Health Insurance (JKN).
DOI: 10.5220/0009567501210126
In Proceedings of the 1st International Conference on Health (ICOH 2019), pages 121-126
ISBN: 978-989-758-454-1
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
121
The research sites in five regions in Indonesia
were in accordance with the Minister of Health
Regulation No. 27 of 2014 concerning Technical
Guidelines for the Indonesian Case-Based Groups
(INA-CBGs) System. This provision was made to
accommodate differences in the cost of distributing
drugs and medical devices in Indonesia.
The selection of provinces is done purposively
based on the regionalization system, namely the
provinces of Jawa Barat, Jawa Timur, Sumatera
Selatan, NTB, Aceh, Sulawesi Utara, Sulawesi
Selatan, Kalsel, Kalteng, Maluku Utara, and Papua.
The selection of Districts/Cities is done purposively
based on urban criteria in provincial capitals,
urban/rural rather than provincial capitals and
underdeveloped / border districts.
Data triangulation is done through the
triangulation of methods and sources of information.
Analysis of RTD results from data and interviews
conducted using the content analysis method and
secondary data analyzed descriptively.
3 RESULT AND DISCUSSION
3.1 Provincial and District/City
Funding Sources for Drugs and
Vaccines
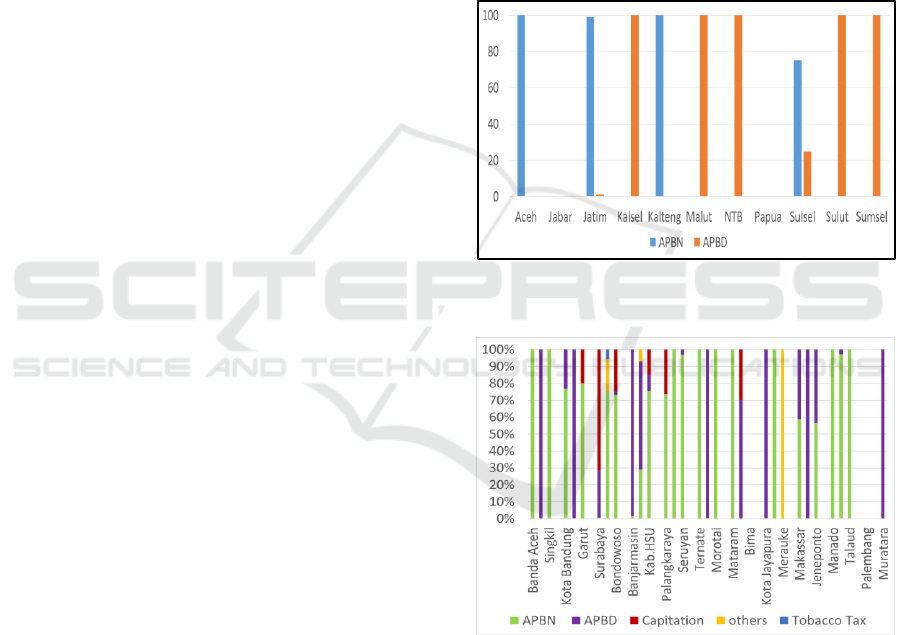
The results of research in 11 provinces show that
2016 drug expenditure uses Regional budget
(APBD) and state budget (APBN) budget sources.
The provinces of Aceh and Kalimantan Tengah
only use the APBN, the provinces of Jawa Timur
and Sulawesi Selatan mostly come from the APBN.
Meanwhile, Kalimantan Selatan, Maluku Utara,
Nusa Tenggara Barat (NTB), Sulawesi Utara, and
Sumatra Selatan Provinces only use the APBD. Jawa
Barat Province has no drug procurement and does not
budget for drug expenditure, the provincial buffer is
obtained from the central government. Drug financing
in provincial and district health offices is sourced
from funds including the state budget, namely the
DAK from Ministry of Health (Directorate General of
Pharmaceutical and Medical Devices), the regional
budget and capitation or other funds.
The variety of health financing budget sources in
Indonesia must be managed properly so that the
funding can complement the needs of each Province.
The central government remains responsible for
fulfilling drug needs in each province, even though
in the JKN system there is a financing model
through capitation and the INA CBGs package.
Obermann in 2018 described financing in the
Philippines during the universal health insurance
period for its citizens. Funding is from a
Government full subsidy program that comes from
the central government budget and sin tax.
(Obermann, 2018).
At the District/City level, spending sources on
medicines in the year 0f 2016 vary, from the State
Budget (APBN), Regional Budget (APBD),
capitation and others. Specifically, in Trenggalek
District, there are spending drug sources that
originate from Tobacco Tax. The composition of
drug expenditure sources in Regencies/Cities can be
seen in figure 2.
Figure 1: Provincial funding sources for drugs and
vaccines.
Figure 2: District/City spending sources in the year of
2016.
The health budget through a tobacco tax as has
been done in the Tranggalek district actually has
been submitted by National Team for the
Acceleration of Poverty Reduction (TNP2K) in 2015
and the same thing also happened in other countries
such as the Philippines. TNP2K states that this is
something that needs to be done as a form of sin tax.
Considering smoking and alcohol is a form of
activity that damages health and can cause illness
ICOH 2019 - 1st International Conference on Health
122
and cause death in the future, it is necessary to do
several strategies including increasing taxes and
using it for health costs as a consequence as well as
efforts to reduce its users, especially among young
people. (National Team for the Acceleration of
Poverty Reduction, 2015).
Capitation funds use policies in several regions, in
general, have already allowed the use of capitation
funds with various special provisions in the region.
3.2 District/City Distribution Cost
Component
The allocation of non-physical DAK in the health
sector in the form of BOK aims to support local
governments in ensuring the availability of quality,
equitable and affordable medicines, vaccines and
medical consumables in government basic health
services. One goal, in particular, is to support the
District/City Health Office in ensuring the
availability of drugs, vaccines and medical
consumables at puskesmas through the provision of
drug and vaccine distribution costs to puskesmas and
the operation of electronic drug and vaccine logistics
information systems at the District/City Pharmacy
Installation. (Ministry of Health, 2016)
The operational policy of the Health Operational
cost (BOK) fund is for the distribution costs of
drugs, vaccines, and medical consumables to be used
to help ensure sufficient quantities of drugs,
vaccines, and medical consumables are available at
the puskesmas. (Ministry of Health, 2016).
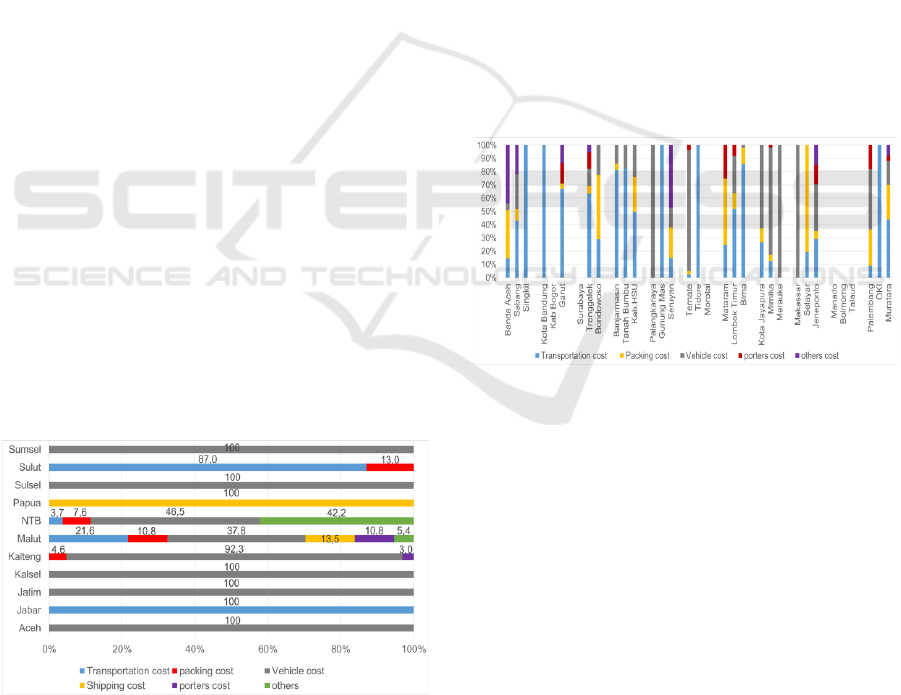
Distribution Cost Components in 11 Provinces of
the study locations indicate that most of the
distribution costs are located in the vehicle cost
component which includes rental costs and/or fuel
costs, or auctions for distribution by third parties.
Figure 3: Province distribution cost component.
The provinces of East Java, Aceh, South
Sumatra, and South Kalimantan use the tendered
system with third parties, including PT POS
Indonesia to distribute to Regencies/Cities, auction
agreements with third parties including packing of
goods (figure 3).
All distribution costs in Papua Province are for the
expedition fee, District/City which is close to the
provincial capital making their own take to the
Provincial pharmaceutical warehouse. Jawa Barat
Province does not have a distribution allocation,
distribution is done by providing an Official Travel
Order (SPPD) for transportation of officers (figure 3).
Since the decentralization system in Indonesia, the
most important thing to do is effective coordination
from the national to the city/district level. Effective
coordination, especially in the effort to manage
budget resources from the central government, the
government and others will support the achievement
of the main objectives of the health system, one of
which is ensuring the availability of medicines for the
community. (Agustina et al., 2018).
The cost component of the District/City
distribution is mainly for the transportation costs of
officers. In Bogor Regency, Surabaya City and
Morotai Regency, Puskesmas take their medicines
themselves to the District/City health office (figure 4).
Figure 4: District/City distribution cost component.
Distribution is one of the important efforts to
ensure the availability of drugs. The amount of
distribution costs required varies greatly, depending
on distance, travel time, regional characteristics,
facilities and types of transportation availability and
transportation to be used. this needs to be taken into
account in the distribution budget preparation. In
addition, there are other budgets that need to be
taken into account, among others, the need for an
insurance budget, both for officers and for collateral
due to loss of goods due to robbery or hijacking
during the trip (Jérôme Dumoulin, Miloud Kaddar &
Germán Velásquez, 1998).
The percentage of distribution costs in 11
provinces is mostly less than 10%, except in South
Kalimantan Province, distribution costs are up to
51.3% compared to provincial health service
Sustainability of Financing to Increase Drug Access and Distribution during National Health Insurance (JKN)
123
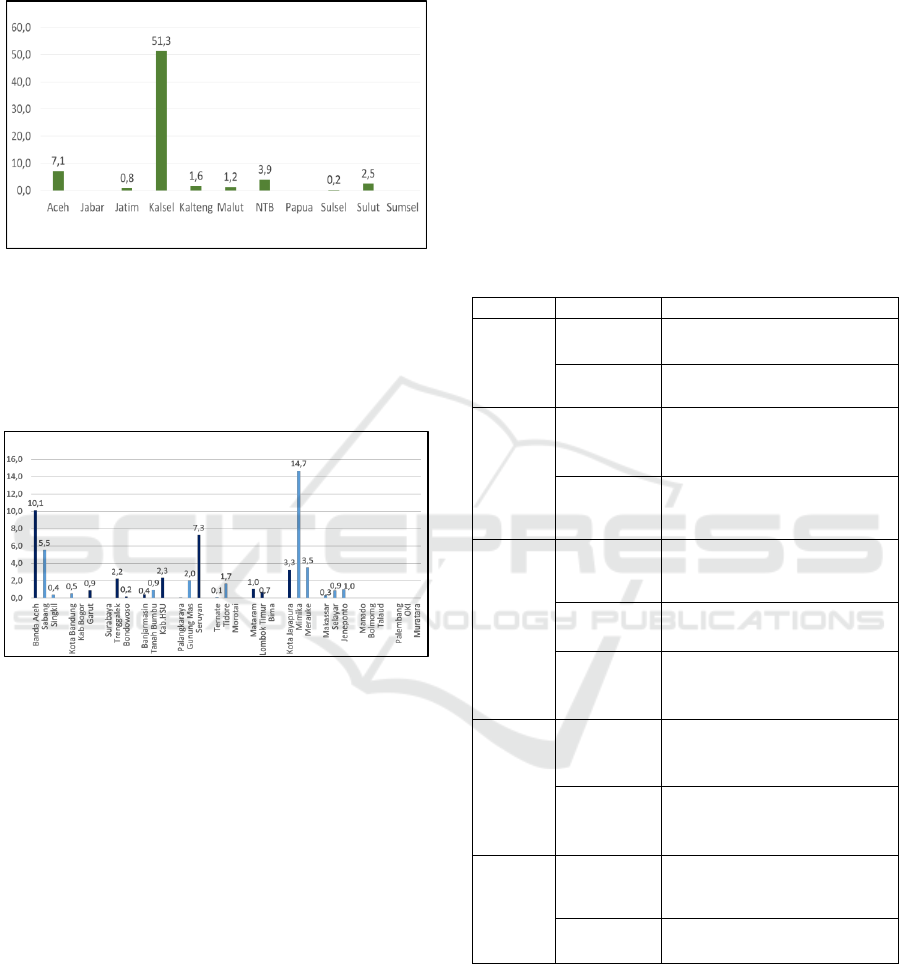
expenditure in 2016. There is no data on the amount
of distribution budget in West Java, Papua, and
South Sumatra Provinces (figure 5).
Figure 5: Percentage of drug distribution costs compared
to provincial health office expenditure in 2016.
The percentage of distribution costs in the
Districts/Cities is also largely less than 10%, except
for Mimika Regency, the comparison of distribution
costs versus drug expenditure is 14.7% (figure 6).
Figure 6: Percentage of distribution costs compared to
District/City health office drug expenditure in 2016.
In Bogor Regency and Surabaya City, there is no
distribution cost data because puskesmas take their
own medicines to the District/City office. The
districts of Banda Aceh, Garut, Trenggalek,
Bondowoso, Banjarmasin, Hulu Sungai Utara,
Seruyan, Mataram, Lombok Timur, and Jayapura
City stated that distribution costs were sufficient,
even though the percentage was less than 10%.
While in Mimika Regency, although more than 10%
is still not sufficient (figure 6).
The amount of distribution cost components
required by each region, especially in Indonesia,
varies greatly and cannot be determined in general,
for example, 10% of drug expenditure. It really
depends on the geographical conditions,
infrastructure and transportation facilities available
and the amount of drug expenditure incurred. 14.7%
of the distribution costs in Mimika, Papua is not
enough to fulfill drug distribution services, while in
other Districts/Cities, they have been able to meet
the needs, although they do not meet 10% of drug
expenditure. This happens because of differences in
the amount of drug expenditure, differences in the
area, differences in the number of health facilities
and differences in geographical conditions.
Geographical conditions, especially in eastern
Indonesia, is one of the challenges in the distribution
process, plus the lack of infrastructure and facilities,
so a good drug distribution system is needed. (Id et
al., 2019).
The mechanism of drug procurement, in general,
is greater with e-purchasing, the rest is by tendered
or direct purchase if the amount is small.
Table 1: Drug purchasing system.
Region
District
I
Jawa Barat
e-purchasing (80%)
tendered (20%)
Jawa Timur
e-purchasing 80-90%,
direct purchasing 10-20%
II
Nusa
Tenggara
Barat
e-purchasing 60% - 80%,
tendered 20% direct purchasing
0-20%
Sumatera
Selatan
e-purchasing
direct purchasing < 5%
tendered < 10%
III
Aceh
e-purchasing 75% -100%
tendered and direct purchasing
<25%
Sulawesi
Utara
e-purchasing 75-80%
tendered ≤ 25%
Sulawesi
Selatan
e-purchasing 70% - 80%
direct purchasing 20% - 30%
IV
Kalimantan
Selatan
e-purchasing 50%-80%
tendered and direct purchasing
20% -50%
Kalimantan
Tengah
e-purchasing 50% -100%
tendered and direct purchasing
0 – 50%
V
Maluku Utara
e-purchasing 90%-100%
tendered and direct purchasing
≤ 10%
Papua
e-purchasing 75%,
direct purchasing 25%
In the era of national health insurance, the cost of
drug distribution is included in the cost of drugs in
the e-catalog system. This needs to be considered
well because if the cost of drug distribution becomes
one component with the cost of the drug, it will
indirectly affect the quality of the drug, if the
distribution costs needed are exceeding the
production costs of drugs ordered. To anticipate this,
ICOH 2019 - 1st International Conference on Health
124

it is necessary to calculate the price of e-catalog
drugs accurately and strengthen the implementation
of the good manufacturer practices (GMP)
monitoring in the pharmaceutical industry which has
won the e-catalog auction. (Id et al., 2019).
The e-purchasing process is an effort to reduce
the drug price in Indonesia, the same thing is done in
India. With the e-purchasing system, the negotiation
process is carried out nationally so it can reduce the
effort and negotiation time in health facilities, and
provide certainty for the pharmaceutical industry in
the production process, as well as large purchases
can increase national drug costs efficiency.
(Ashigbie, Azameti, and Wirtz, 2016).
The health insurance system will increase the
role of pharmaceuticals in the effort to provide
quality services that have proven to be cost-
effective. At present, the central and regional
governments still finance the procurement of drugs
for the public sector in first-level health facilities
and drug drugs programs, in the future the
availability of drugs in health facilities will be the
responsibility of BPJS and health facilities in
collaboration with BPJS as holders of health
insurance programs in Indonesia, while the central
and regional governments are responsible for public
health programs (National Team for the
Acceleration of Poverty Reduction, 2015).
To improve budget efficiency and use of health
resources as needed, the health system needs to be
implemented policies that support the use of
medicines and other health resources that are
appropriate and clinically proven that involve
various sectors of drug management, ranging from
the pharmaceutical industry to patients, for example
by providing an incentive or reward and punishment
system for the parties involved in it (Wagner, Quick,
and Ross-degnan, 2014).
4 CONCLUSIONS
In several provinces still rely on the state budget
(APBN) as the main source of funding for drug
distribution. Costs for purchasing drugs are sourced
from the state budget and regional budget, as well as
capitation in several regions. For drugs, because they
are included in the price of the e-catalog to the
health office, there is generally no problem in the
distribution costs of drugs. Most of the costs incurred
by the DHO are for repacking and transporting the
sending staff as well as increasing endurance costs.
The endurance enhancement costs are allocated
especially for regions that do not specifically have
distribution costs because they are not allowed in the
budget system. Distribution work is considered to be
a task so no additional costs can be given.
ACKNOWLEDGMENTS
The authors thank the Head of Centre for Health
Resources and Services Research and Development
along with the management team for conducting
research activities ranging from providing budgets to
administration and licensing processes so that they
can produce information that can be useful for
policymakers.
REFERENCES
Agustina, R. et al. (2018) ‘Review Universal health
coverage in Indonesia: concept, progress, and
challenges’. doi: 10.1016/S0140-6736(18)31647-7.
Ashigbie, P. G., Azameti, D. and Wirtz, V. J. (2016)
‘Challenges of medicines management in the public
and private sector under Ghana ’ s National Health
Insurance Scheme – A qualitative study’, Journal of
Pharmaceutical Policy and Practice. Journal of
Pharmaceutical Policy and Practice, pp. 1–11. doi:
10.1186/s40545-016-0055-9.
Id, R. W. et al. (2019) ‘Use of medicine pricing and
reimbursement policies for universal health coverage
in Indonesia’, pp. 1–20. doi: 10.1371/journal.pone.
0212328.
Jérôme Dumoulin, Miloud Kaddar & Germán Velásquez
(1998) Guide to drug financing mechanisms. World
Health Organization, England.
Ministry of Health (2006) "Ministry of Health of the
Republic of Indonesia Regulation No.189 / Menkes /
SK / III / 2006 concerning National Medicines Policy"
Ministry of Health (2016) "Ministry of Health of the
Republic of Indonesia Regulation of the No.71 of
2016 concerning Technical Guidelines for the Use of
Non-Physical Special Allocation Funds for Health in
2017 Budget Year"
National Team for the Acceleration of Poverty Reduction
(2015) The Road to National Health Insurance (JKN).
Jakarta: secretariat of the Vice President of the
Republic of Indonesia.
Obermann, K. (2018) ‘The role of national health
insurance for achieving UHC in the Philippines : a
mixed methods analysis’, Global Health Action.
Taylor & Francis, 11(1). doi: 10.1080/16549716.2018.
1483638.
Presidential Regulation of Republik Indonesia No. 72 of
2012 concerning the National Health System
Wagner, A. K., Quick, J. D. and Ross-degnan, D. (2014)
‘Quality use of medicines within universal health
Sustainability of Financing to Increase Drug Access and Distribution during National Health Insurance (JKN)
125

coverage : challenges and opportunities’, 14(1), pp. 1–
6. doi: 10.1186/1472-6963-14-357.
Yuniar, Yuyun (2017). Distribution Availability, And
Drug and Vaccine Service In Facing The 2019
Universal Health Coverage Research. Report Study.
Centre for Health Resources and Services Research
and Development. National Institute of Health
Research and Development. Ministry of Health.
Jakarta
ICOH 2019 - 1st International Conference on Health
126
