
Effect of the Fractional Distillation on an Increment Patchouli
Alcohol Content in Patchouli Oil
Yuliani Aisyah
1,2
, Sri Haryani Anwar
1
and Yulia Annisa
1
1
Agricultural Product Technology, Agriculture Faculty, Universitas Syiah Kuala, Banda Aceh, Indonesia, 23111
2
PUI - Atsiri Research Center, Universitas Syiah Kuala, Banda Aceh, Indonesia, 23111
Keywords: Patchouli oil, fractional distillation, patchouli alcohol, boiling point.
Abstract: Indonesia is one of the patchouli oil producers in the world, however, the problem is the quality of patchouli
oil, especially patchouli alcohol content that are still below the required standard. One of the methods that can
be used to increase the content of patchouli alcohol is fractional distillation method. This research aims to
know the influence of the initial concentration of patchouli alcohol and height of column against increment
of patchouli alcohol content in patchouli oil. The experimental design which used was Complete Randomized
Design (CRD) consist of two factors, first factor namely the initial concentration of patchouli alcohol (C1 =
31,11%, C2 = 32,83%, and C3 = 33,61%) and second factor is height of column (H1 = 25 cm and H2 = 45
cm). Analysis of variance shows that the height of vigreux column has a real influence against the increased
levels of patchouli alcohol. The highest levels of patchouli alcohol (83,86%) obtained from the residue
fraction of distillation with 31.11 % initial concentration of patchouli alcohol and 45 cm height of column.
The higher levels of patchouli alcohol in patchouli oil residue fraction, the higher specific gravity and the
refractive index, and solubility in ethanol will be easier. The result shows that this sample have 1.013 specific
gravity, clear in ethanol at 1:5 and have 1.5166 refractive index.
1 INTRODUCTION
Patchouli (Pogostemon cablin Benth) is one of the
plants that produce an essential oil known as the
Patchouli oil. Patchouli comes from a family of
Lamiaceae, the order of Lamiales and Class of
Angiospermae. There are several types of Patchouli
in Indonesia, such as Pogostemon cablin Benth, or
widely known as Aceh Patchouli (Nilam Aceh),
which has the oil content of 2.5-5%. Furthermore,
the Pogostemon heyneanus which is known as Java
Patchouli (Nilam Jawa) with the oil content of 0.5-
1.5%, and Pogostemon hortensis also known as Soap
Patchouli with the oil content of 0.5-1.5% (Rukmana,
2003).
According to Aisyah et al. (2008), there are 15
identified chemical constituents of Patchouli oil. The
constituents with the highest percentage are patchouli
alcohol (32.60%), δ-guaiene (23.07%), α-guaiene
(15.91%), seychellene (6.95%) dan α-
patchoulene (5.47%). These five components are also
similar to the result of Corine and Sellier (2004).
The patchouli alcohol (PA) is one of the quality
parameters of patchouli oil. Patchouli alcohol is an
oxygenated sesquiterpene that has a boiling point of
140 ºC at 8 mmHg pressure, the molecular weight of
224 and a molecular formula of C
15
H
26
O (Bulan et al.,
2000). According to the international standard, the
best quality of patchouli oil is the one with patchouli
alcohol content at least 38% (Essential Oil
Association of USA, 1975), and 31% (SNI 06-2385-
2006). The patchouli oil produced in Indonesia
relatively has a low content of patchouli alcohol
which is < 30%. This is because the postharvest
handling before distillation is not conducted very
well, the distillation process is not optimal (simple
method and equipment, and short distillation time),
and because of the material source. Therefore, the
parameter of patchouli alcohol content needs to be
improved to expand the market.
All this time the farmers only capable to produce
the oil with patchouli content of 26-28%, while the
distillation industry that uses the stainless-steel
distillation equipment can produce the oil with
patchouli alcohol content up to 31-35% (Sarwono,
1998).
Several types of research have been conducted to
improve the patchouli alcohol content in patchouli oil
80
Aisyah, Y., Anwar, S. and Annisa, Y.
Effect of the Fractional Distillation on an Increment Patchouli Alcohol Content in Patchouli Oil.
DOI: 10.5220/0009957100800085
In Proceedings of the 2nd International Conference of Essential Oils (ICEO 2019), pages 80-85
ISBN: 978-989-758-456-5
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
by using different method, for instance the fractional
distillation (Bulan et al., 2000; Harfizal, 2003;
Yanyan et al., 2004), rotary evaporator with
fractionation temperature control (Suryatmi, 2008),
cellulose acetate membrane (Aisyah et al., 2010),
vacuum fractionation distillation (Aisyah et al., 2013;
Isaroiny and Mitarlis, 2005), a combination of
fermentation, delignification and distillation methods
(Muharam et al., 2017). The result showed that the
increase of patchouli alcohol content varies depends
on the method used.
Based on the boiling point, constituents of
patchouli oil have boiling point as follows: patchouli
alcohol (140°C at 8 mmHg), eugenol (252.66 °C at
760 mmHg), benzaldehyde (178.07 °C at 760
mmHg), cinnamic aldehydes (251.00 °C at 760
mmHg) and cadinene (274 °C at 760 mmHg)
(Guenther, 1949). The difference in the boiling point
leads the components to be separated by the
fractionation distillation process. To provide a
thorough result and prevent component damage due
to the impact of temperature on ordinary fractionation
distillation, the process will be accompanied by
vacuum fractionation distillation.
This research aims to optimize the vacuum
fractional distillation by considering the factor of
initial patchouli alcohol of patchouli oil and factor
of fractionational distillation column used in this
process. It is assumed that these two factors can
produce a higher percentage of final patchouli alcohol
than initial patchouli alcohol from patchouli oil.
2 MATERIAL AND METHOD
2.1 Material
The material used in this research is patchouli oil
from Meukek, Pasie Raja, and Panjupian Sub-district
of Aceh Selatan Regency. The equipment used is a
series of vacuum fractionation distillation equipment
consisting of 500 ml round neck flasks, vigreux
columns, thermometers, condensers, 3 heart-shaped
flasks, vacuum pumps, pans, and hot plates. Quality
analysis is using Gas chromatography-mass
spectrometry Shimadzu GCMS-QP 2010S, GC-QP
2010S, pycnometer, analytical scale, test tubes, drop
pipettes and Abbe refractometers.
2.2 Method
Vacuum Fractional Distillation Process (Modified by
Aisyah, 2008). The fractional distillation process of
patchouli oil is using a series of vacuum fractionation
distillation equipment which is accompanied by a
vacuum pump. The patchouli oil used is 300 ml. The
distillation was done at ±2 KPa (± 15.001 mmHg)
pressure and temperature of 30-190 °C. The
distillation was conducted until there are no more
distillate drops on the heart-shaped flask. The sample
of patchouli oil is analyzed by using GC-MS before
fractionation. The residue from the fractional
distillation then was analyzed to determine the final
patchouli alcohol content. Furthermore, the residue
with the highest content of patchouli alcohol was
analyzed for refractive index and solubility in water.
3 RESULT AND DISCUSSION
3.1 Chemical Constituent of Patchouli
Alcohol
The Chromatogram (Figure 1) represents the analysis
result of chemical constituent using GC-MS on three
patchouli oil before fractionation, and the chemical
constituent components in patchouli oil that is above
1% can be seen in Table 1.
Table 1: Chemical constituent of Patchouli Oil
Chemical constituent Meukek
Pasie
Raja
Panju
pian
β-Patchoulene 1,62 1,76 1,73
2,4-Diisopropenyl-1-
methyl-1-vinyl-
cyclohexane
1,00 1.02 1,20
β-Caryophyllene 2,87 2,77 3,06
ߙ-Guaiene
17,39 16,61 18,10
Seychellene 4,68 4,27 4,67
α-Patchoulene 6,89 6,46 6,65
Alloaromadendrene 2,82 2,81 3,32
Δ -Guaiene 21,45 20,23 20,08
1H-
Cycloprop[e]azulen-
4-ol, decahydro-
1,1,4,7-tetramethyl
4,03 5,35
Patchouli alcohol 31,11 32,83 33,61
2H-Pyran-2,4(3H)-
dione, 3-acetyl-6-
methyl
1,12
Effect of the Fractional Distillation on an Increment Patchouli Alcohol Content in Patchouli Oil
81

Figure 1: Chromatogram of GC-MS result of Patchouli Oil:
(a) Meukek Patchouli Oil, (b) Pasie Raja Patchouli Oil, (c)
Panjupian Patchouli Oil
Based on Figure 1, it shows that each oil has the
same chromatogram pattern but has different peak
heights, which means that each patchouli oil has
different percentage of each of the different chemical
constituent components. The five highest constituents
are patchouli alcohol, Δ-guaiene, α-guaiene, α-
patchoulene, seychellene, and β-
carryophyllene, with a different percentage in each
patchouli oil (Table 1).
The difference in the percentage of each oil
caused by some factors, namely genetic (type),
cultivation, environment, postharvest and postharvest
handling (Irawan, 2010). It is assumed that the most
influencing factor from all of the mentioned factors is
the factor of the distillation process that leads to the
difference in the chemical constituent of patchouli oil
before the fractionation. It has been mentioned before
that the oils come from three different distillation
location. Thus, the distillation was conducted
differently.
The result from GC-MS analysis on Table 1 is
slightly different from the research by Corine and
Sellier (2004), who identified 4 new constituents
which are γ-gurjunene, germacrene D, aciphyllene
and 7-epi-α-selinene. Whereas in this research, the
result from analysis on oil before fractionation found
the component of γ-gurjunene and germacrene A.
Besides the factors mentioned above, it is assumed
that this difference is due to the method used in
analyzing using GC-MC is also different.
3.2 Patchouli Alcohol Content
Vacuum fractional distillation which was conducted
at ±2 KPa pressure can produce an average of 3
fractions, which are 2 distillates and 1 residue. Each
fraction was produced from a different temperature.
Based on the research of Aisyah (2008) we know that
the residue fraction from fractional distillation as a
higher content of patchouli alcohol than the other
fraction. Therefore, this research is analyzing the
patchouli alcohol using GC on residue fraction.
The result from GC analysis shows that the
patchouli alcohol ranged from 31.98% to 83.86%
with the average of 55.17%. The result from analysis
of variance shows that the height of Vigreux column
has a significant effect (P ≤ 0,01) on the increase of
patchouli alcohol content in patchouli oil.
Meanwhile, the initial content of patchouli alcohol
and interaction between two factors has no significant
effect (P>0,05) on the increase of patchouli alcohol
content in patchouli oil. The influence of Vigreux
column height on the increase of patchouli alcohol
content can be seen in Figure 2.
LSD
0.05
test result shows that the 45 cm column
can increase the patchouli alcohol content in
patchouli oil and higher than the use of a 25 cm
column. Based on Figure 2, the increase of patchouli
alcohol content on the 45 cm column is different
significantly with the 25 cm column.
The column is used to separate the vapor from
liquid compound which has a similar boiling point
(<20°C). The barrier (tray/plate) in the fractionation
column causes vapors with the same boiling point
will both evaporate or compounds with low boiling
points that will continue to rise until finally condenses
and descends as a distillate. Meanwhile, if the
compounds with higher boiling points have not
reached the boiling point value, they will drip back
into the distillation flask, which eventually will reach
the boiling point value if the heating continues. The
compound will evaporate, condense and drop/drip as
a distillate.
A
B
C
ICEO 2019 - 2nd International Conference of Essential Oil Indonesia
82
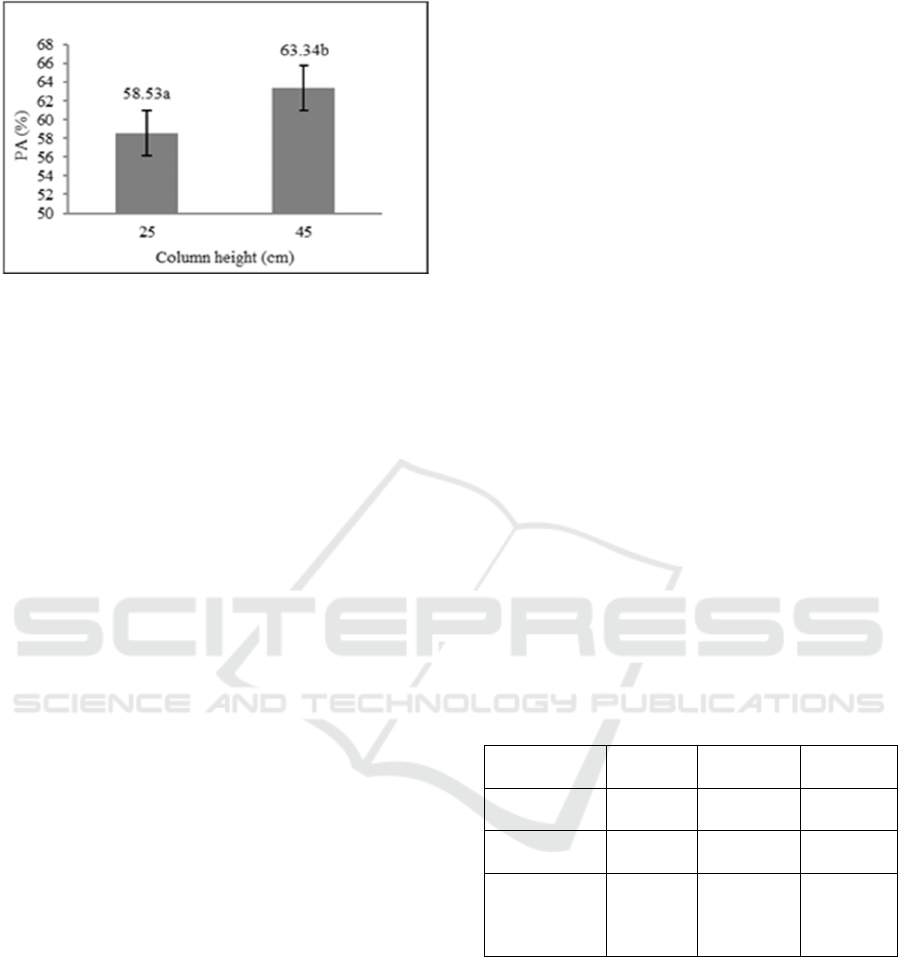
Figure 2: The effect of vigreux column height on the
increase of patchouli alcohol (PA) content in residue
fraction (LSD
0.05
= 4,06, CV = 20,18%, values that followed
by the same letter show no significant difference).
The components of a substance undergoing the
fractionation distillation process will experience
direct contact in the column. The fractionation
column (vigreux column) contains a tray that serves
as a component selection media. During the
distillation process, the components in the oil will
evaporate according to the boiling point of each
component and pass through the trays in the column.
The farther the tray is from the heat source, the lower
the temperature of the tray.
It is assumed that the components that can rise to
the top of the 45 cm column and becomes distillate
are only those who have boiling point lower than
patchouli alcohol. This is due to the low temperature
of the tray at the top of the column. Hence, only the
components with a low boiling point that can
maintain the form of gas after hitting the tray.
Whereas the temperature difference between base and
top of 25 cm column is not too far away that makes
the components with a similar boiling point with
patchouli alcohol also evaporate and becomes
distillate.
According to Geankoplis (1983), condensation or
the process of gas turn into liquid occurs when
saturated gas touches the solid that has a temperature
below gas temperature. This conversion makes the
components that have a low boiling point fall back
down to the base of the column.
Geankoplis (1983) also stated that if the
component is in liquid form when passing through the
tray, then the components will fall to the previous
tray. Meanwhile, the components in the gas form will
keep drove off to the next tray where the components
will be having more contact with the liquid that
coming down from the tray above it. Under these
conditions, the concentration of the component with
a low boiling point will increase in the vapor and
decrease in the liquid which descends towards the
bottom of the fractionation column. This statement
implies that the fractionation column, especially the
tray in the column, can affect the chemical
composition of the distillate fraction and the residue
from the fractionation distillation.
According to Ma’mun and Maryadhi (2008),
patchouli alcohol has a relatively higher boiling point
than other components in patchouli oil. The content
that was obtained in this research is lower than the
content of patchouli alcohol from the research of
Ma’mun and Maryadhi (2008) which is about 91.5%.
This is due to the difference of pressure used by those
researchers with the pressure used in this research.
This research is using the pressure of ± 2 KPa because
it was the minimal pressure that can be reached by the
vacuum pump. Meanwhile, Ma’mun and Maryadhi
(2008) used the pressure of 0 cmHg (similar with 0
KPa) that caused the boiling point of those
components can be reduced further and the separation
occurs more easily without having to experience
overheating which allows decomposition.
The result of the research is also contrary to the
research from Aisyah et al. (2008). It is assumed that
the difference was due to the same cause which is the
difference in the condition of the vacuum fractional
distillation process. The comparison of results from
several types of research about vacuum fractional
distillation of patchouli alcohol can be found in Table
2.
Table 2: The comparison of research result of vacuum
fractional distillation of patchouli oil
Condition
Aisyah
(2008)
Ma’mun
(2008)
This
research
Pressure 4 mmHg 0 mmHg 2 KPa (15
mmHg)
Temperature 90-135
ºC
150-180 ºC 140-190
ºC
Highest
patchouli
alcohol
content
87.36 % 91.5 % 83.86 %
3.3 Physical Properties of Residue
Fraction of Patchouli Oil
Analysis of physical properties was performed on
specific gravity, solubility in ethanol and refractive
index. The sample is residue fraction from treatment
which has initial content of 31.11% with 3 different
columns. The chosen sample is the sample from the
treatment that produced a residue fraction with a
patchouli alcohol content higher than other samples.
Effect of the Fractional Distillation on an Increment Patchouli Alcohol Content in Patchouli Oil
83

3.4 Specific Gravity
Specific gravity is the result of the ratio comparison
between oil weight and water weight at the same
volume and temperature (SNI, 2006). According to
Gunther (1949), this parameter is essential in finding
the foreign matter in a liquid or the shifts that may be
affecting the quality of the oil. The result from the
analysis of specific gravity (Table 3) shows that the
specific gravity of the sample goes beyond the
standard that has been determined by the Indonesian
National Standard (SNI) which is about 0.950-0.975.
This suggests that the residue fraction of patchouli oil
from the treatment of 25 cm and 45 cm column cannot
be sell as crude oil. However, the residue fraction can
be applied as a material in derivative products from
patchouli oil.
Table 3: Specific gravity and refractive index of residue
fraction of patchouli oil
Sample
Specific
Gravity
Refractive
Index
Without fractionation 0.953 1.5070
25 cm column height 1.005 1.5156
45 cm column height 1.013 1.5166
Table 3 shows the increase of the specific gravity
of patchouli oil before and after fractionation
(control). This increase influenced by the components
in the oil. According to Rizal (2010), specific gravity
represents the comparison between heavy fractions
and light fractions contained in the oil. The heavier
fractions contained a higher specific gravity. The
heavy fractions are influenced by the length of the
molecular chain of a compound contained in the oil.
Patchouli oil is a compound with a molecular
formula of C
15
H
26
O. Hence, this compound has a
relatively long molecular chain which caused the oil
to dominated by high specific gravity patchouli
alcohol components. The result is shown in Table 3.
The patchouli oil before fractional distillate only
contains 31.11% of patchouli alcohol and a specific
gravity of 0.953, while the residue fraction of
patchouli oil which has been fractionated by 25 cm
column has 75.14% of patchouli alcohol and a
specific gravity of 1.005. Furthermore, the residue
fraction of patchouli oil which has been fractionated
by 45 cm column has 83.86% of patchouli alcohol
and a specific gravity of 1.013.
3.5 Refractive Index
The refractive index is the ratio of the velocity of
light in air to its velocity in the examined substance at
a certain temperature (Armando, 2009). According to
Guenther (1949), the index of refraction value of
patchouli oil or other essential oil can be determined
by using Abbe refractometer. The result from the
analysis of the refractive index (Table 3) shows the
treatment of a 45 cm column with the initial content
of 31.11% resulting in the highest refractive index of
1.5166. Guenther (1949) explained that the value of
the specific gravity of essential oil will affect the
refractive index value. As can be seen at Table 3,
Patchouli oil which has not been fractionated and
with a patchouli alcohol content of 31.11% with a
specific gravity value of 0.953, has a refractive index
value of 1.5070, where this value is much lower than
after fractionation with a 45 cm column height of
1.5166. This is in accordance with the statement from
Armando (2009) who stated that the more
components with a long chain-like sesquiterpene or
sesquiterpene or oxygen clusters components
contained, the density of essential oil medium will
increase. Hence, the light will be harder to refract and
the refractive index value will be higher.
3.6 Solubility in Ethanol 90%
The solubility of patchouli oil in ethanol is one of the
examinations of patchouli oil quality based on
physical properties. This test is conducted to
determine the purity of essential oil.
Table 4: Residue fraction of patchouli oil solubility in
Ethanol 90%
Without
fractionation
25 cm column
height
45 cm column
height
1 : 5 Turbid 1 : 4 Turbid 1:3 Turbid
1 : 6 Turbid 1 : 5 Turbid 1:4 Turbid
1 : 7 Turbid 1 : 6 Turbid 1:5 Soluble
1 : 8 Soluble 1 : 7 Soluble 1:6 Soluble
Based on Table 4, it can be seen that the patchouli
oil residue fraction resulting from the K
1
T
2
treatment
is soluble at a ratio of 1: 5 (1 ml of oil and 5 ml of
ethanol). The treatment shows a clear solution at the
lowest ratio compared to other treatments, even
clearer than the raw material (control) which is
soluble at a ratio of 1:8. According to Guenther
(1949), the solubility of oil in alcohol is determined
by the type of chemical components contained in
essential oil. In general, essential oils which contain
oxygenated terpene compounds will be more soluble
in alcohol compared to essential oils containing non-
oxygenated terpene components. This is because of
the non-oxygenated terpene compounds which are
ICEO 2019 - 2nd International Conference of Essential Oil Indonesia
84

nonpolar compounds that do not have functional
groups. Thus, it is difficult to react with alcohol.
Patchouli alcohol is a component in patchouli oil
which is included in an oxygenated terpene
compound and has a functional group. Therefore,
patchouli oil which has a higher level of patchouli
alcohol such as the residual fraction resulting from
the K
1
T
2
treatment will be more soluble in alcohol
compared to other treatment.
4 CONCLUSION
The height of vigreux column used in vacuum
fractionation distillation has a significant effect on the
increase of patchouli alcohol levels in patchouli oil
residue fraction, while the initial patchouli alcohol
levels did not affect the increase in patchouli alcohol
levels in patchouli oil residue fraction. The value of
specific gravity and refractive index from the fraction
of residual fractionation result of patchouli oil is
higher than patchouli oil before fractionation so that
the solubility in ethanol will be easier. The highest
alcohol content of patchouli was obtained from
fractionation distillation using a column height of 45
cm which was 83.86%.
REFERENCES
Aisyah, Y., Hastuti, P., Sastrohamidjojo, H., and Hidayat,
C. 2008. Komposisi Kimia dan Sifat Antibakteri
Minyak nilam (Pogostemon cablin). Majalah Farmasi
Indonesia, 19 (3), 151-156.
Aisyah, Y., Hastuti, P., Hidayat, C., and Sastrohamidjojo,
H. 2010. Peningkatan Kadar Patchouli Alkohol Minyak
Nilam (Pogostemon cablin Benth) dengan
Menggunakan Membran Selulosa Asetat. Jurnal
Agritech, 30(3), 184-191.
Aisyah, Y., S.H. Anwar., and Y, Annisa. 2013. Increment
of patchouli alcohol in patchouli oil by vacuum
distillation fraction method. Proceedings of The 3rd
Annual International Conference Syiah Kuala
University (AIC Unsyiah) In conjunction with The 2nd
International Conference on Multidisciplinary
Research (ICMR).
Armando, R. 2009. Memproduksi 15 Jenis Minyak Atsiri
Berkualitas. Penebar Swadaya, Jakarta.
BSN (Badan Standardisasi Nasional). 2006. SNI No. 06-
2385-2006. http://sisni.bsn.go.id (10 Mei 2012).
Bulan, R. 2004. Esterifikasi Patchouli Alkohol Hasil Isolasi
dari Minyak Daun Nilam (Patchouli Oil). Jurusan
Kimia Fakultas Matematika dan Ilmu Pengetahuan
Alam Universitas Sumatera Utara.
Corine, M.B., Sellier, N.M., 2004. Analysis of The
Essential Oil of Indonesian Patchouli (Pogostemon
cablin Benth.) Using GC/MS (EI/CI). J. Essent. Oil
Res., 16, 17-19.
Essential Oil Association of USA. 1975. EOA
Spesifications and Standard. EOA USA. New York.
Geankoplis, G.J. 1983. Transport Process and Unit
Operation. Second Edition, Allyn and Bacon, Inc,
Boston, London, Sydney, Toronto.
Guenther, E. 1949. Essential Oils : Volume II. Van
Nostrand Reinhold Company, New York.
Harfizal. 2003. Penerapan teknologi distilasi vakum untuk
meningkatkan mutu minyak nilam. Prosiding Seminar
Teknologi untuk Negeri.
Irawan, B. 2010. Peningkatan Mutu Minyak Nilam dengan
Ekstraksi dan Destilasi Pada Berbagai Komposisi
Pelarut. Tesis. Magister Teknik Kimia Universitas
Diponegoro, Semarang.
Isaroiny, R., Mitarlis. 2005. Peningkatan Kadar Patchouli
Alkohol pada Minyak Nilam (Pogostemon cablin
Benth) dengan Metode Distilasi Fraksinasi Vakum.
Berk. Penel. Hayati, 10, 123–127.
Ma’mun., Adhi Maryadhi. Isolasi Patchouli Alkohol dari
Minyak Nilam untuk Bahan Referensi Pengujian dalam
Analisis Mutu. Bul. Littro, 19(1), 95 – 99.
Muharam, S., Lela, M. Y., and Iim, S. R. 2017. Peningkatan
Kualitas Minyak Nilam (Pogostemon Cablin Benth)
menggunakan Kombinasi Metode Fermentasi,
Delignifikasi dan Destilasi. Jurnal Kimia Valensi, 3 (2),
116-121.
Rizal, S. 2010. Kajian Proses Penyulingan Minyak Nilam
Menggunakan Sistem Distilasi Air. Fakultas Teknologi
Pertanian IPB, Bogor.
Rukmana, R. 2003. Nilam: Prospek Agribisnis dan Teknik
Budidaya. Kanisius. Yogyakarta.
Sarwono, B. 1998. Budidaya Nilam di Purbalingga. Trubus
343-Th XXIX-Juni 1998. 77-78.
Suryatmi, R.D. 2008. Fraksinasi minyak nilam. Prosiding
Konferensi Nasional Minyak Atsiri.
Yanyan, F.N., Zainuddin, A., Sumiarsa, D. 2004.
Peningkatan kadar patchouli alkohol dalam minyak
nilam (patchouli oil) dan usaha derivatisasi komponen
minornya. Jurnal Perkembangan Teknologi TRO, 16,
72-78.
Effect of the Fractional Distillation on an Increment Patchouli Alcohol Content in Patchouli Oil
85
