
Antioxidant of Total Phenolic from Saputangan Leaves
(Maniltoa grandiflora (A. Gray) Scheff)
Jhon Patar Sinurat
1
, Reh Malem Br Karo
2
, Saadah Siregar
1
, Romauli Teresia Marbun
1
, Fahma
Shufyani
1
1
Faculty of Pharmacy Institut Kesehatan Medistra Lubuk Pakam Sudirman street No. 38 Lubuk Pakam, Medan, Indonesia
2
Faculty of Pharmacy Universitas Prima Indonesia Belanga Street No.1 Ayahanda Medan, Indonesia
Keywords: Antioxidant, Total Phenolic, Thin Layer Chromatography and Spectrophotometer of UV-Visible
Abstract: Phytochemical screening test used 5% FeCl
3
reagent showed extract become to black extract that
saputangan leaves contained phenolic compound. Saputangan leaves powder was macerated with methanol
and got macerate as 100.84 g. Macerate was dissolved with aquadest to remove lipid in saputangan leaves.
First partition was used ethyl acetate to get solid extract as 37.03 g. Second Partition was used n-hexane to
get solid extract as 18.25 g. Total phenolic was analysed on plate of thin layer chromatography with used
chloroform and methanol with 70:30 comparison. Total phenolic has 3 spots that have an Rf of 0.44 ; 0.29
and 0.22. Total phenolic absorbance measured at 516 nm wavelength using a UV-Visible
spectrophotometer. Total phenolic solutions were soluted in concentrations of 10, 25 and 50 ppm using
methanol p.a as solvent. The absorbance obtained at all three concentrations was 0.202; 0.195 and 0.175.
Based on the linear regression equation Y = 1,191 X + 31.87, the IC
50
value for the total phenolic was 15.22
ppm. IC
50
value of 15.22 ppm can be categorized as a total phenolic compound as a strong antioxidant.
1 INTRODUCTION
The development of plant production is increasing
by the community who more understanding about
the benefits of medicinal plants. In developing
countries, 65%–80% of population depends upon
herbal medicines for primary health care (Oladele
and Ayoola., 2015). Different categories of bioactive
compounds are being isolated and characterized
since the middle of 19th century. Most of these
compounds are used as raw material for new
medicines or as an active ingredient of existing
medicines. Herbal medicines provide rich amount of
tannins, alkaloids, flavonoids, phenolic compounds,
and so forth, so these can be used in the treatment of
several degenerative disorders (Ali et al, 2015).
Plants are rich and valuable resources of bioactive
phenolic. They can be utilized in various fields such
as antioxidant, antimicrobial, anti-inflammatory,
antitumor, antiviral, analgesic and antipyretic
(Salinas et al 2017).
Saputangan Plants (Maniltoa grandiflora (A.
Gray) Scheff) is a type of plant that belongs to the
genus Maniltoa and Fabaceae family. Saputangan is
usually made as ornamental plants that can reduce
pollution by absorbing pollutants such as carbon
monoxide (Hidayati et al., 2016). In English the
handkerchief plant is named as Dove Tree, Ghost
Tree, Handkerchief Tree. Historically, the origin of
this Saputangan plants came from Fiji. The
distribution of saputangan is from the areas of Fiji,
Indonesia, Papua New Guinea, Solomon Islands,
Tonga and the United States. This plant is a tree
with high 5 to 15 m. Stems upright, round,
simpoldial branching and brown. Leaves in the form
of complex leaves, pinnate leaf reinforcement, oval
flat edges, pointed edges and base of leaves. Leaf
length of 7 to 14 cm and width of 3-8 cm with stem
length between 1 - 1.5 cm and green. It has malae-
shaped compound flowers and is located under the
leaves and ends of tree trunks. The shape of the
flower stalk is round with length as 1-2 cm and
green. Cup shaped petals, oval sheath, loose flower
crowns and yellow. The fruits of this plant are pods.
Kidney shaped seeds, black and small in size. The
types of roots include taproots and brownish white
(Health Department, 2015).
Phytochemical screening method is done by
checking at the color testing reaction using a color
reagent. The important thing that plays an important
536
Sinurat, J., Br Karo, R., Siregar, S., Marbun, R. and Shufyani, F.
Antioxidant of Total Phenolic from Saputangan Leaves (Maniltoa grandiflora (A. Gray) Scheff).
DOI: 10.5220/0009974405360542
In Proceedings of the International Conference on Health Informatics and Medical Application Technology (ICHIMAT 2019), pages 536-542
ISBN: 978-989-758-460-2
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

role in phytochemical screening is the selection of
solvents and extraction methods. Phytochemical
screening of simplicia powder and samples in wet
form includes checking the content of alkaloids,
flavonoids, terpenoids, tannins and saponins
according to procedures that have been carried out
by Harbone (Subramanian et al., 2016). The
effective parameters in these extraction methods are
the type and polarity of solvents and their ratio, time
and temperature of extraction and moreover
chemical composition and physical characteristics of
the samples (Garcia et al, 2015). Maceration is a
stepwise solid-liquid extraction method which is
carried out by leaving the solids submerged in a
solvent. The process of immersion in an effort to
extract a substance from this natural material can be
done without heating (at room temperature), by
heating or even at boiling temperatures. After
filtering, the residue can be extracted again using a
new solvent. A new solvent in this case does not
necessarily mean different substances from the
previous solvent but can be a solvent of the same
substance. This process can be repeated several
times as needed. If maceration is done with a water
solvent, a further extraction process is needed, it is
the water phase extraction obtained with organic
solvents. If maceration is directly carried out with
organic solvents, the extracted filtrate is collected
into one, then evaporated or distilled (Kristanti et al.,
2018). Partition is the process of separation to obtain
components of solutes from their mixtures in solids
using an appropriate solvent. It can also be defined
as the dispersion of the chemical component of the
extract which has been dried in an appropriate
solvent based on the solubility of the chemical
component and undesirable substances such as
insoluble salts. This extraction operation can be
carried out by stirring the solid suspension in a
container with or without heating (Jiao et al, 2015).
Thin Layer Chromatography on a larger layered
plate, usually 5 x 20 cm, 10 x 20 cm, or 20 x 20 cm.
Usually it takes 30 minutes to an hour of
development. In essence, TLC involves two phases,
namely the stationary phase or layer properties, and
the mobile phase or mixture of developer solvents.
The stationary phase can be a fine powder that
functions as an absorbent or buffer surface for a
liquid layer. The mobile phase can be almost any
kind of solvent or a mixture of solvents. The
selection of the right mobile phase is a very
important step for the success of the analysis with
TLC (Sri Atun, 2016). Phenol compounds are the
main class of antioxidants in plants. The content of
phenolic compounds is widely known as a free
radical terminator and in general the content of
phenolic compounds is positively correlated to
antiradical activity. Phenolic compounds are easily
found in plant parts such as stems, leaves, flowers,
and fruit. The large variety of groups which may be
substituted in the main framework of phenol causes
a wide structural variation in phenolic compounds.
There are more than 8000 types of compounds
included in the group of phenolic compounds and
whose structures are known include flavonoids,
simple monocyclic phenols, phenyl propanoids,
polyphenols (lignin, melanin, tannin) and phenolic
quinones (Marinova et al., 2015).
Phenolics are one of the major and diverse group
of active compounds in the plants which have at
least one aromatic ring and one or more hydroxyl
groups in their structures (Gharaati et al, 2017). In
terms of biogenetics, phenol compounds are
basically divided into two main types. The first is
the phenol compound derived from the shikimat
pathway and the second is the phenol compound
derived from the acetate-malonate pathway. Another
class of phenol compounds derived from a
combination of these two biosynthetic pathways is
the flavonoid compound (Kristanti et al., 2018).
Antioxidants are inhibitors of oxidation reactions
due to free radicals that can cause damage to
unsaturated fatty acids, cell wall membranes, blood
vessels, DNA bases, and lipid tissue, causing
disease. A plant has antioxidant activity if it contains
compounds that are able to ward off free radicals
such as phenols and flavonoids. Free radicals occur
due to complex chemical processes in the body that
can damage the body's immune system. If there are
excess free radicals in the body will be able to attack
anything that can have implications for the
emergence of various degenerative diseases,
therefore the formation of free radicals must be
prevented or served with antioxidants (Widyastuti,
2015).
Recent research conducted by Sinurat et al.
(2018) showed that the methyl gallate compound
isolated from saputangan leaves had a very strong
antioxidant ability with IC
50
value of 16,136 mg/ml.
Lubis et al. (2018) who isolated phenolic
compounds in the form of methyl gallate from
jengkol skin (Archidendron jiringa) which is a
Fabaceae family which is proven to have very strong
antioxidant power. Previous research was also
conducted by Dzoyem et al. (2017) regarding the
antioxidant, antimicrobial and cytotoxic activity of 8
compounds isolated from Entada abyssinica
(Fabaceae) where there are 4 types of phenolic
compounds that can act as antibacterial. Based on
Antioxidant of Total Phenolic from Saputangan Leaves (Maniltoa grandiflora (A. Gray) Scheff)
537

this description, the researcher was interested in
testing the antioxidant activity of the total phenolic
compounds of the saputangan leaves using the
DPPH method (2,2-diphenyl-1- picrilhidrazil).
2 METHODS
This research was conducted in the natural product
of organic chemistry laboratory, pharmacy faculty of
Institut Kesehatan Medistra, Lubuk Pakam. The
study was conducted in the period April-August
2018. The Saputangan leaves obtained from the
environment around the Universitas Sumatera Utara.
Materials: Saputangan leaves powder, Methanol p.a.,
Ethyl acetate, n-Hexane, Chloroform p.a., Aquadest,
reagent of 5% FeCl
3
and DPPH (2,2-diphenyl-1-
picrilhidrazil). Equipment: Macerator, Separate
Funnel (Schoot Duran), Rotary evaporator
(Heidolph), Steaming waterbath (Memmert), TLC
plate, Chamber, Incubator (Memmert) and UV-Vis
Spectrophotometer (Shimadzu). Saputangan leaves
that have dried and blend become powder.
This research was carried out sequentially in
laboratory with the research scheme. Process started
from maceration and screening test, then continued
to evaporate solvent. Solid extract is soluted by
water to remove the lipid. Then filtrated the fraction
that soluted in water. Filtrate is partitied with ethyl
acetate conducted with n-hexane by separate funnel.
TLC is done to analysis of total phenolic compound
and measure of antioxidant activity. The scheme of
research is showed in Figure 1.
Figure 1: Scheme of Research.
Phytochemical Screening: This research was
conducted in the laboratory of organic chemistry of
natural materials, Department of Chemistry, Faculty
of Mathematics and Natural Sciences to determine
the presence of phenolic compounds in the leaves of
the Saputangan plant. A preliminary test was carried
out, phytochemical screening where 10 g fresh
leaves of saputangan plant that had been blended
with a blender macerated with methanol and then
filtered. The filtrate was tested by adding 3 drops of
5% FeCl
3
reagent solution, forming a black
precipitate if saputangan extract is positive
contained phenolic compound (Eko, 2015).
Maceration of Saputangan Leaves:
Sample as
1000 g of Saputangan leaves powder which had
been dried and finely macerated for ± 24 hours with
methanol as much as 5 liters at room temperature.
Macerate was filtered and a extract of saputangan
leaves was obtained. Maceration was repeated using
methanol as a solvent until the methanol extract
obtained gave a negative test result with 5% FeCl
3
reagent. The methanol extract obtained was
concentrated by rotary evaporator at a temperature
of 60
o
C with a rotation of 80 rpm. In Figure 2a is
shown the saputangan leaves. Saputangan is a tree
with high 5 to 15 m. Stems upright, round,
simpoldial branching and brown. Leaf length of 7 to
14 cm and width of 3-8 cm with stem length
between 1 - 1.5 cm and green.
Partition of Saputangan Leaves: Patition of the
saputangan was carried out on the distilled water
filtrate in a 500 ml separating funnel using ethyl
acetate solvent so that the bottom layer was obtained
in the form of distilled water and the top layer was in
the form of ethyl acetate. Then the ethyl acetate
layer is taken and continued with repeated partitions
of the aquades filtrate. The ethyl acetate extract
obtained was concentrated by a rotary evaporator at
60
o
C with a rotation of 40 rpm and evaporated until
the solvent evaporated. Then partitioned repeatedly
with n-hexane and evaporated by rotary evaporator
at 60
o
C with a rotation of 30 rpm. The partition
process is shown in Figure 2b and 2c.
(a) (b) (c)
Figure 2: (a) Saputangan Leaves, (b) Partition in Ethyl
Acetate, (c) Partition in n-hexane.
Thin Layer Chromatography: Total phenolic
obtained from the partition extraction process were
ICHIMAT 2019 - International Conference on Health Informatics and Medical Application Technology
538

analyzed using the Thin Layer Chromatography
(TLC) method using the Merck 60F254 silica gel
stationary phase and the Chloroform:Methanol at a
ratio of 90:10, 80:20, 70:30 and 60:40 v/v. To see
the change in elucidation, thin layer chromatograms
were marked with upper and lower limits using a
pencil. The total phenolic compound is dropped at
the lower limit of the thin layer plate, then put into a
chamber that containing the eluent and allowed to
move to the upper limit. Thin layer chromatograms
were irradiated with ultraviolet light to see the spots
of the compound, then marked with a pencil and
calculated the Rf value. Furthermore, the thin layer
was fixed with a 5% FeCl
3
solution producing a
black spot on the thin layer chromatogram showing
positive containing phenolic compounds. Observed
the color of the stain that arises and calculate the
price of Rf obtained. Rf range from 0.00 to 1.00.
Separate components are good if the value of Rf is
different from at least 0.1. The same thing is done in
each eluent comparison used to determine the results
of the separation of the thin layer chromatogram.
Stains that arise are calculated using the factor
retention formula as follows: Rf formula is stain
distance to the lower limit per eluent distance to the
lower limit.
Antioxidant Test:
0.3 mM DPPH solution was
prepared by dissolving 2.957 mg of DPPH powder
in methanol p.a in a 25 mL measuring flask, then
homogenized so that the solution to be formed was
violet. Total phenolic prepared in 100 ppm as main
solution, by dissolving 1 mg of total phenolic with
methanol p.a solvent in a 10 ml measuring flask.
Then the 100 ppm main solution soluted in variation
of solution with concentrations of 10, 25 and 50
ppm. 1 ml of DPPH 0.3 mM was added with 2.5 ml
of methanol p.a as a blank solution. 1 ml of 0.3 mM
DPPH solution was added with 2.5 ml of total
phenolic with a concentration of 10 ppm,
homogenized in a test tube and left for 30 minutes in
a dark room. After that measured absorbance with a
maximum wavelength of 516 nm. The same work
procedure was carried out to test the antioxidant
total phenolic compounds by concentrations of 25
ppm and 50 ppm.
3 RESULTS
Phytochemical screening is using 5% FeCl
3
reagent
where previously the sample was dissolved with
methanol solvent in repeatedly. In this case, the
extract was became black precipitate after dropped
5% FeCl
3
whereas the extract was previously green.
The screening result is tested in test tube. The black
precipitate is meant saputangan leaves contained
phenolic. The following will be displayed screening
result in Figure 3a. Maceration process is treated to
the powder sample of saputangan leaves in
macerator. Maceration is treated repeatly to
maximize the extract that resulted. Sample as solid
extract was macerated in methanol solvent was
obtained at 100.84 g. This method was carried out
by inserting suitable plant powders and solvents into
a tightly closed inert container at room temperature.
The principle of maceration method is based that
samples soaked using organic solvents will break
down the walls and cell membranes due to pressure
differences found outside and inside the cell so that
secondary metabolites contained in the cytoplasm
will dissolve into organic solvents. The extraction
process is stopped when an equilibrium is reached
between the concentration of the compound in the
solvent and the concentration in the plant cell
(Yeon-Ju et al., 2015).
After maceration, a partition was carried out
using ethyl acetate to obtain a solid extract of 37.03
g. The last partition was carried out using n-hexane
to partitied the non polar compound from phenolic
compound. Finally, the solid extract after the last
partititon is 18.25 g. The extract saputangan leaves
from the partition was contained total phenolic
because it reacted positively to the FeCl
3
reagent
when we have screening again. In the liquid-liquid
partition process, two phases of solution have
differences soluble in solubility. The shaking of the
separating funnel during partition aims to expand the
contact surface area between the immiscible
solvents. The solvent requirement for the partition
method has polarity which is suitable for the
extracted material and must be separated after
shaking. Extract of total phenolic after many process
as black extract is shown in Figure 3b.
Thin layer chromatography (TLC) analysis was
performed on total phenolic compounds obtained
using chloroform : methanol eluent 70:30 v/v. Based
on the results of TLC analysis, it can be concluded
that the total phenolic compounds contained 3 polar
phenolic compounds. The following figure is
displayed of the results of TLC analysis of total
phenolic compounds. Chromatogram of TLC is
shown in Figure 3c.
Antioxidant of Total Phenolic from Saputangan Leaves (Maniltoa grandiflora (A. Gray) Scheff)
539
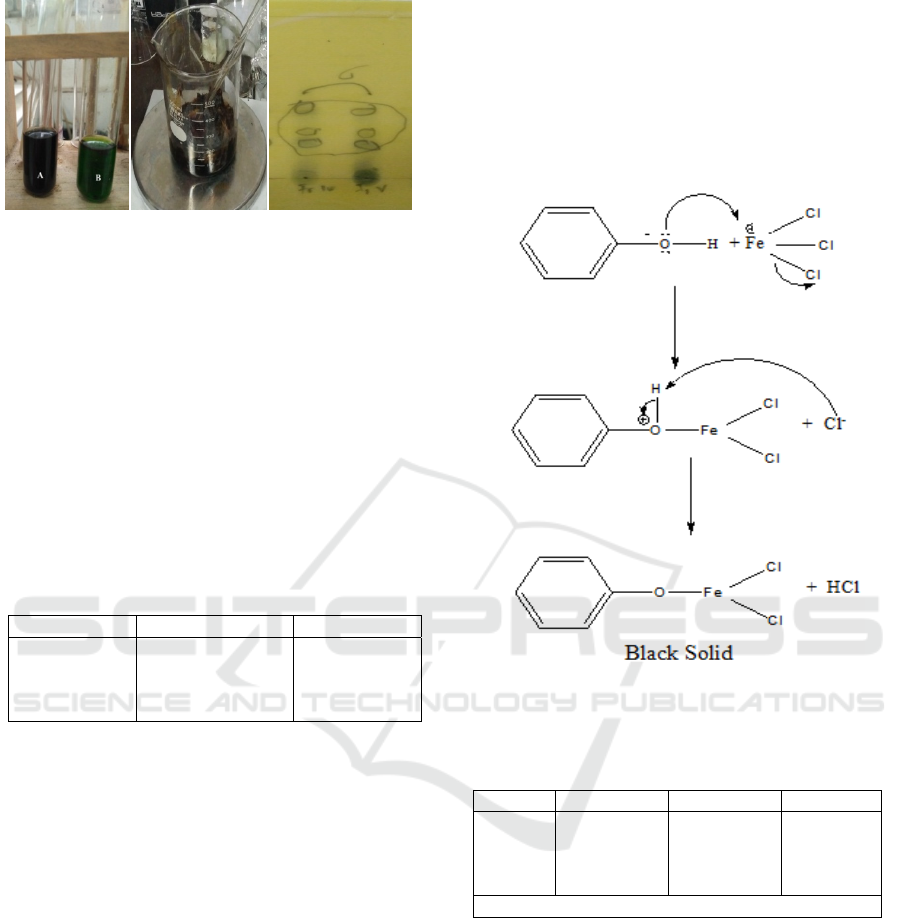
(a) (b) (c)
Figure 3: (a) Saputangan leaves extract + 5% FeCl
3
, (b)
Extract of Total Phenolic, (c) TLC analysis of
total phenolic.
Total phenolic compounds of saputangan leaves
were tested for antioxidant activity by the free
radical DPPH method to obtain IC
50
values by a UV-
Visible spectrophotometer at a maximum
wavelength of 516 nm. The concentration is
prepared in many variation, it is 10, 25 and 50 ppm.
The absorbance is showed in instrument then
converted the absorbance in percentage scale. The
measurement is displayed in table 1.
Table 1: Measurement of total phenolic absorbance.
Concentration Absorbance % Absorbance
Blank
10 ppm
25 ppm
50 ppm
0.802
0.202
0.195
0.175
-
74.83 %
75.72 %
78.19 %
4 DISCUSSIONS
The green extract became a black extract indicating
that extract contained phenolic compounds. It
happened because the oxygen group which is bound
as a hydroxy releases a pair of free electrons to bind
FeCl
3
so that the H group that is bound as hydroxy
will be released and form an aromatic compound
with bound to FeCl
2
as a black precipitate and HCl.
Figure 4 is regarding the mechanism of reaction.
Thin layer chromatography analysis was
performed on total phenolic compounds using
chloroform eluent: methanol 70:30 v/v, so that 3
spot spots were obtained. Stain spot is calculated
according the Retention Factor formula. Retention
factor of Stain spot 1 is 0.44, stain spot 2 is 0.29 and
stain spot 3 is 0.20. Total phenolic compounds of
saputangan leaves were tested for antioxidant
activity by the free radical DPPH method to obtain
IC
50
values by a UV-Visible spectrophotometer at a
maximum wavelength of 516 nm. The equation Y =
ax + b is used to obtain the value of IC
50
by entering
the value 50 as the Y axis, so value x will be
obtained that will represent the value of IC
50
.
Statistical calculation is showed in Table 2 and
conducted to complete linear regression equation.
Figure 4: The mechanism of phenolic with FeCl
3
.
Table 2: Statistical Calculation.
Note : X = Concentration (ppm)
Y = Absorbance (%)
The “a value” obtained from the statistical
formula is 1.191. This a value will be used as the x-
axis in the linear regression equation. The “b value”
obtained from the statistical formula is 31.87. This b
value is 31.87 will be used as the constant in the
linear regression equation. The linear regression
equation is Y = ax + b. The “a and b value” are
substituted to the linear regression equation as IC
50
value to be Y = 1.191 X + 31.87. If the
concentration is increased, percentage of absorbance
X Y XY X
2
0 0 0 0
10 74.83 748.3 100
25 75.72 1893 625
50 78.19 3909.5 2500
ΣX= 85 ΣY= 228.74 ΣXY=6550.8 ΣX
2
=3225
ICHIMAT 2019 - International Conference on Health Informatics and Medical Application Technology
540
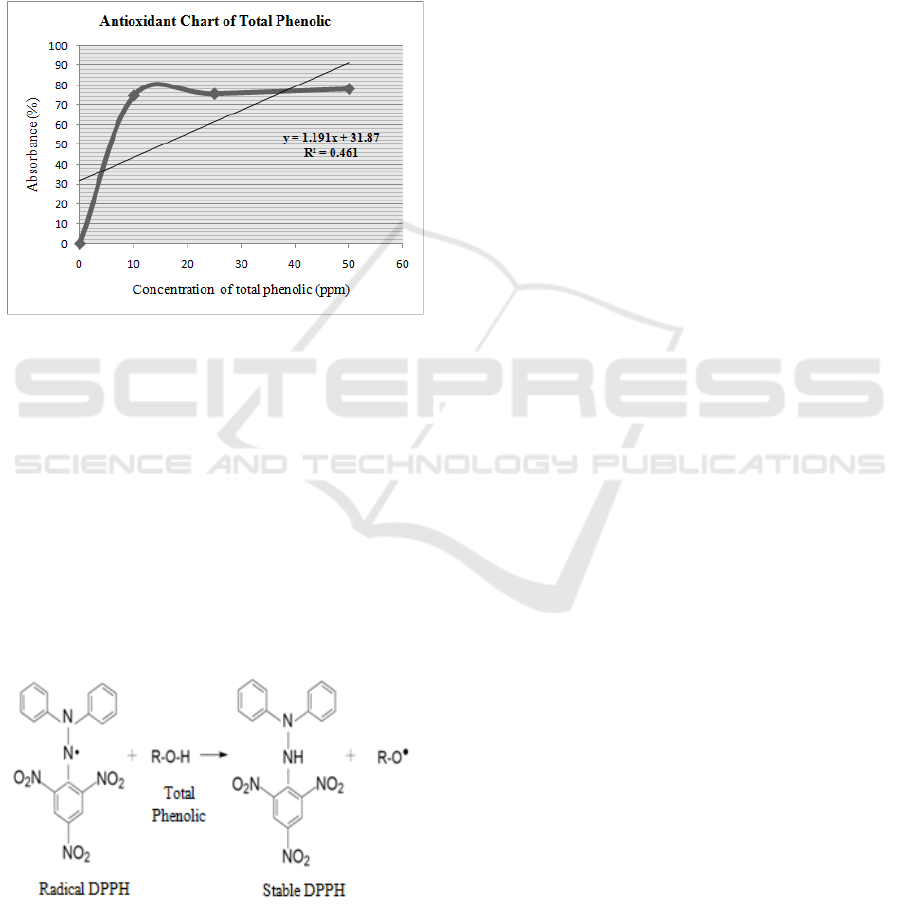
will increase too. Based on the linear regression
equation got IC
50
values as 15.22 ppm. Based on the
literature it can be stated that if the IC
50
value
produced is below 50 ppm which indicates that the
total phenolic has a very strong antioxidant activity.
According to the equation, The R
2
value is 0.461.
The correlation between concentration and
percentage (%) of absorbance can be seen in Figure
5.
Figure 5: Chart of Antioxidant.
In the antioxidant test, absorbance of DPPH
radical is followed by a reversal of absorbance at the
maximum wavelength that occurs due to radicals by
antioxidants (AH) or reactions with radical species
(R.) which are marked by changes the color became
pale yellow color, data often given as IC
50
is an
antioxidant needed for 50% of DPPH radical
reduction in a certain period of time (15-30 minutes)
(Pokorny et al, 2016). The mechanism between
DPPH radical and total phenolic will be displayed in
Figure 6. The Hydrogen from phenolic compound
will stable the DPPH radical, so that phenolic can
act as antioxidant.
Figure 6: Absorbance mechanism of DPPH radical.
5 CONCLUSION
After maceration and partition, a total phenolic
compound is obtained from saputangan leaves as
18.25 g. Results of thin layer chromatography
analysis of total phenolics using the chloroform :
methanol as eluent showed that total phenolic has 3
spots that have an Rf of 0.44 ; 0.29 and 0.22. The
total phenolic compound is able to act as a strong
antioxidant by having an IC
50
value of 15.22 ppm.
This antioxidant test was carried out using a DPPH
(2,2-diphenyl-1- picrilhidrazil) which was measured
using a UV-Visible spectrophotometer at a
wavelength of 516 nm.
ACKNOWLEDGEMENTS
Thank you to The Institut Kesehatan Medistra
Lubuk Pakam for the laboratory facilities and
finance provided for this study and research.
REFERENCES
Ali, K., Artun, FT., Ozcan, G., Melikoglu, G., Anil, S.,
Sutlupinar, N., 2015. In vitro evaluation of antioxidant
activity of some plant methanol extracts,
Biotechnology and Biotechnological Equipment, vol.
6, no. 6, pp. 1184–1189.
Dzoyem, JP., Melong R., Tsamo, AT., Tchinda, AT.,
Kapche, DG., Ngadjui, BT., Mcgaw, LC., Eloff, JN.,
2017. Cytotoxicity, Antimicrobial and Antioxidant
Activity of Eight Compounds Isolated from Entada
abyssinica (Fabaceae). Bio Med Central Research
Notes, 10: 1-6.
Eko, B,M., 2015. Phytochemical Screening and Total
Flavonoid Content in the Fruit Carica pubescens
Lenne & K. Koch in the Bromo, Cangar and Dieng
Plateau Regions. Phytochemical Screening, 5(2) : 73-
82
Garcia, SP., Morales, SA., Segura, CA., Fernández, GA.,
2015. Phenolic-compound extraction systems for fruit
and vegetable samples. Molecules;15(12):8813-8826
Gharaati, S,. Kargar, H., Falahati, AM., 2017,
Tetrahydropyranylation of alcohols and phenols
catalyzed by a new multiwall carbon nanotubes-bound
tin(IV) porphyrin. Journal of the Iranian Chemical
Society ;14(6):169-1178
Health Department, 2014. Indonesia Health Profile in
2015. Jakarta :Kemenkes RI.
Hidayati, N., Mansur, M., Juhaeti, T., 2016. Variation of
Carbon dioxide (CO
2
) uptake of tree species in
"Ecopark", Cibinong and their links to the potential for
greenhouse gas mitigation. Pusat Penelitian Biologi
LIPI. Cibinong.
Antioxidant of Total Phenolic from Saputangan Leaves (Maniltoa grandiflora (A. Gray) Scheff)
541

Kristanti, AN., 2018. Phytochemical Textbooks. Surabaya:
Universitas Airlangga Press.
Jiao, T., Li, C., Zhuang, X., Cao, S., Chen, H., Zhang, S.,
2015. The new liquid-liquid extraction method for
separation of phenolic compounds from coal tar.
Chemical Engineering Journal ;266:148-155.
Lubis, MY., Siburian, R., Marpaung, L., Simanjuntak, P.,
Nasution, MP., 2017. Methyl Gallate from Jiringa
(Archidendron jiringa) and Antioxidant Activity.
Asian Journal of Pharmaceutical and Clinical
Research, 11: 346-350.
Marinova, D., Ribarova, F., Atanassova, M., 2015. Total
Phenolics and Total Flavonoids in Bulgarian Fruits
and Vegetables. J Univ Chem Technol Metal, 40 : Hlm
255-260.
Oladele, JO., and Ayoola, GA., 2015. Antioxidant activity
among selected medicinal plants combinations (multi-
component herbal preparation), International Journal
of Research in Health Science, vol. 3, no. 2, pp. 526–
532.
Pokorny, J., Yanishlieva, N., Gardon, M., 2016.
Antioxidants In Food, Practical Application, 1-123,
wood Publishing Limited. Cambridge, England.
Salinas, MY., García, SC., Ramírez-Díaz, JL., La Alemán-
de, TI., 2017. Phenolic compounds in maize grains
and its nixtamalized products. In: SotoHernández M,
Palma-Tenango M, GarciaMateos MDR, editors.
Importance and Applications. Phenolic Compounds
Natural Sources. IntechOpen.
Sinurat, JP., Lenny, S., Sebayang, F., 2018. Isolation of
Phenolic Compound and Antioxidant from Saputangan
Leaves (Maniltoa grandiflora (A. Gray) Scheff).
International Journal Science and Technology
Engineering, 5: 60-65.
Sriatun, 2016. Method of Isolation and Identification of
Structure of Natural Organic Compounds. Journal of
Borobudur Cultural Heritage Conservation,, 8(2) : 53-
61.
Subeki, 2014. Effect of Cooking Method on Antioxidant
Content of Several Kinds of Vegetables as well as
Absorption and Retention in Experimental Rats.
Postgraduate Program. Institut Pertanian Bogor.
Subramanian, R., Chandra, M., Yogapriya, S., Aravindh,
D., Ponmurugan, K., 2016. Isolation of Methyl Gallate
from Mango Twigs and its Anti-biofilm Activity.
Journal of Biologically Active Products from Nature,
6:5-6 : 383-392.
Widyastuti, N., 2015. Measurement of Antioxidant
Activity with CUPRAC, DPPH, and FRAP Methods
and Correlation with Phenols and Flavonoids in Six
Plants, Bachelor of Science Thesis. Bogor. Institut
Pertanian Bogor.
Yeon-Ju, L., Jeong-Woo, L., Dong-Geun, L., Hyi-Seung,
L., Jong, SK.., and Jieun, Y., 2015. Cytotoxic
Sesquiterpenoids Isolated from the Marine Sponge
Scalarispongia sp. J. Molecular Science, 15(11):
20045-20053.
ICHIMAT 2019 - International Conference on Health Informatics and Medical Application Technology
542
