
Modification of Mung Bean Starch by Annealing Treatment and
Acetylation
Azis Boing Sitanggang
1,2
, Patricia Sani
3
and Titri S. Mastuti
3
1
Department of Food Science and Technology, IPB University. Kampus IPB Darmaga Bogor 16680, West Java, Indonesia
2
Southeast Asian Food and Agricultural Science and Technology (SEAFAST) Center, IPB University,
Kampus IPB Darmaga Bogor 16680, West Java, Indonesia
3
Department of Food Technology, Pelita Harapan University, MH Thamrin Boulevard 1100,
Tangerang 15811, Banten, Indonesia
Keywords: Mung Bean, Mung Bean Starch, Dual Modification, Annealing, Acetylation, Modified Starch,
Physicochemical Characteristics of Modified Starch.
Abstract: Mung beans are mainly composed of starch (25-30%). Mung bean starch which found naturally has low
stability during processing and heat sensitive. The purpose of this research was to produce mung bean starch
with higher resistance to heat and higher resistance to enzyme digestion, with combination of annealing and
acetylation modification. Modified starch with 60
o
C of annealing temperature resulted in highest crystallinity.
Additionally, with 20% of acetic acid anhydride concentration and 15 minutes acetylation reaction time
resulted in acetyl percentage and substitution degree that met FDA requirement. Dual modification of
annealing and acetylation with chosen treatments as mentioned, was analysed further to determine starch
content, moisture content, amylose and amylopectin content, swelling power and solubility properties, colour
test, resistant starch content, XRD profile, FTIR profile, pasting properties, and starch granule morphology.
1 INTRODUCTION
Mung bean belongs to Fabaceae family. This bean
can be found easily in Asia, Australia, New Zealand,
and Africa (Yang et al., 2018). Mung bean has
±7.91% water content, ±24.08% protein content,
±1.55% fat content, ±2.87% ash content, ±2.20%
fiber content, and ±25.73% starch content
(Moongngarm, 2013).
Mung bean has bioactive compound like tannin,
phytic acid, flavonoid, phenolic acid, and other
organic acids. These bioactive compounds in mung
bean has positive effects on health, such as free
radical scavenger, detoxification, anti-bacterial,
prevent diabetes, and prevent cancer (Ganesan & Xu,
2018). Mung bean production in Indonesia reach
370,000 tons, this amount was not balanced by its
production which reach 303,000 tons (Kementerian
Pertanian, 2018). Therefore, mung bean was used as
ingredient in starch production to increase its
economic value.
Mung beans mainly composed of starch (25-
30%). However, mung bean starch which found
naturally has low stability and low heat resistance
(Phrukwiwattanakul et al., 2014). Therefore,
modification was needed to alter functional properties
of starch and make it applicable to certain food
industries.
Starch modification can change its polymer,
structure, and functional properties, to increase its
function in food industries or non-food industries
(Lopez et al., 2010). However, study about starch
modification using combination of annealing and
acetylation, and its effect on physicochemical
properties on mung beans starch has not been done.
The purpose of this research was to produce mung
beans starch with higher resistance to heat and higher
resistance to enzyme digestion, with combination of
annealing and acetylation modification. Moreover,
this research was done to determine the effects of
selected treatment to mung beans starch
physicochemical properties, with annealing heating
temperature, acetic acid anhydride concentration, and
acetylation reaction time as factors.
10
Sitanggang, A., Sani, P. and Mastuti, T.
Modification of Mung Bean Starch by Annealing Treatment and Acetylation.
DOI: 10.5220/0009977100002833
In Proceedings of the 2nd SEAFAST International Seminar (2nd SIS 2019) - Facing Future Challenges: Sustainable Food Safety, Quality and Nutrition, pages 10-19
ISBN: 978-989-758-466-4
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

2 MATERIALS AND METHODS
2.1 Materials and Equipment
The materials used in this research were mung beans
obtained from Plaza Baru Ciledug market. Distilled
water (Amidis), acetic acid anhydride, pure glucose,
HCl, NaOH, anthrone reagent, iodine, KI, KOH,
H2SO4 (Merck, EMD Millipore Corp.), pure
amylose, phosphate buffer, α-amylase enzyme,
pepsin enzyme, β-amylase enzyme (Sigma-Aldrich,
EMD Millipore Corp.), ethanol (Smart-Lab, PT
Smart Lab), aquadest, and phenolphthalein indicator
were also used in this research.
The equipment used in this research were beaker
glass, Erlenmeyer flask, volumetric flask, Mohr
pipette, graduated cylinder (Iwaki), blender (HR
2071/20, PT Philips), thermometer (ASTM 12C,
BRAND, Ltd.), oven (UNB 500, Memmert, Ltd.),
cabinet dryer (Rekayasa Wangdi), heater (Cimarec),
analytical balance (Pioneer), refrigerator (Tipe SDC
1000, PT Sanden), vortex (Tipe 37600 Mixer,
Barnstead Thermolyne Corp.), spectrophotometer
UV-Vis (Genesys 10S UV-VIS, Thermo Fisher
Scientific Inc.), pH meter (Tipe 744, Metrohm, Ltd.),
waterbath (WB 14, Memmert, Ltd.), burette
“BRAND”, centrifuge (Z 206 A, Hermle Labor
Technik, Inc.), chromameter (CR-400, Konica
Minolta, Inc.), X-Ray Diffraction (MiniFlex, Rigaku
Corp.), Scanning Electron Microscope (Quanta 650
FEG, Thermo Fisher Sientific, Inc.), Fourier
Transform Infrared Spectroscopy (IRPrestige-21,
Shimadzu Corp.), Rapid Visco Analyzer (RVA-4,
Newport Corp.), spoon, glass rod, spatula, filter cloth,
evaporating dish, test tube, test tube clamp, tray,
desiccator, filter paper, magnetic stirrer, stopwatch,
reflux, quartz cuvette, dropping pipette, bulb pump,
micropipette, metalized plastic, silica gel, aluminium
foil, and centrifuge tube.
2.2 Starch Production
Starch production was done based on Abdel-Rahman
et al. (2008) research. Decorticated mung beans were
rinsed with streaming water to get rid of impurities.
Then, cleaned mung beans was soaked for 2 hours,
and the water was discarded. Afterward, distilled
water was added to mung beans with 1:3 (mung
beans: water) ratio, and crushed with blender within
3 minutes. Next, obtained mushed mung beans was
filtered used 60 mesh filter cloth. Filtration process
produced residue and filtrate. Distilled water was
added to the residue and filtrated again two times,
while the filtrate was settled for 2 hours. Precipitation
process produced precipitate and supernatant. The
supernatant was discarded and the precipitate was
dried at 40
o
C for 15-20 hours using cabinet dryer.
Dried precipitate was crushed using dry blender
within 1 minute, then sieved with 60 mesh sieve. This
powder is mung beans starch that had to be stored in
refrigerator in ± 5
o
C until further analysis.
2.3 Preparation of Annealed Starch
Annealing treatment was done by mixing mung beans
starch with distilled water in 1:2 (mung beans starch:
water) ratio. Then, the slurry transferred to 250 ml
beaker glass and covered with aluminium foil. The
beaker and its content was soaked in water bath at
40°C/ 50°C /60°C for 6 hours. After cooling down,
the slurry was centrifuged at 5,000 rpm for 10
minutes. Centrifugation process produced precipitate
and supernatant. The supernatant was discarded,
while the precipitate was washed with distilled water
and filtered with filter paper. The precipitate was
washed to dissolve impurities and help filtering
process. Next, the precipitate was dried with oven at
40°C for 15-20 hours.
2.4 Preparation of Annealed Starch
Mung beans starch produced from selected annealing
treatment was mixed with distilled water with 4:9
(annealed mung beans starch: water) ratio. Then the
slurry was stirred using magnetic stirrer at 25°C for
60 minutes. pH of the slurry was set to 8 with 3%
NaOH solution. Then, acetic acid anhydride (density
= 1,082g/ml) with 10%, 15%, or 20% concentration
based on sample weight (g) was added slowly to the
slurry, while still keeping pH range between 8-8.4
with 3% NaOH solution. Reaction was settled at 25°C
for 5, 15, or 25 minutes after adding acetic acid
anhydride. After that, pH was set to 4.5 with 1 N HCl.
Then, the slurry was filtered with filter cloth to obtain
precipitate and supernatant. The supernatant was
discarded, while the precipitate was washed two
times with distilled water, to get rid of impurities and
HCl residue. Next, the precipitate washed once with
95% ethanol to get rid of acetic acid anhydride
residue in starch. Washed precipitate then dried at
45°C in oven for 24 hours.
2.5 Experimental Design
Experimental design was applied to annealed and
acetylated starch. Obtained data was tested using
SPSS on acetylated starch. Experimental design used
in annealed starch was completely randomized design
Modification of Mung Bean Starch by Annealing Treatment and Acetylation
11
with one factor, three replication, and three level of
heating temperature which is 40°C, 50°C, and 60°C.
Experimental design used in acetylated starch was
completely randomized design with two factor, two
replication, and three level of acetic acid anhydride
concentration which is 10%, 15%, and 20% along
with three level of acetylation reaction time which is
5, 15, and 25 minutes.
2.6 Analysis Methods
Analysis done in this research were starch yield
(Ratnayake et al., 2007), starch content (Ezeigbo et
al., 2015), moisture content (AOAC, 2005), acetyl
percentage and degree of substitution (Colussi et al.,
2015), amylose and amylopectin content
(Abeysundara et al., 2015), solubility properties and
swelling power (Zaman et al., 2015), colour test
(Nadir et al., 2015), pasting properties, resistant
starch content (AOAC, 2000), starch granule
morphology (SEM), XRD profile, and FTIR profile.
3 RESULTS AND DISCUSSION
3.1 Mung Bean Starch Characteristics
Based on identification test, it was confirmed that
mung bean used as main ingredient to produce starch
is Vigna radiata (L.) R.Wilczek. This mung bean was
processed to produce starch that will be analyzed
further. Results of starch analysis can be found in
Table 1.
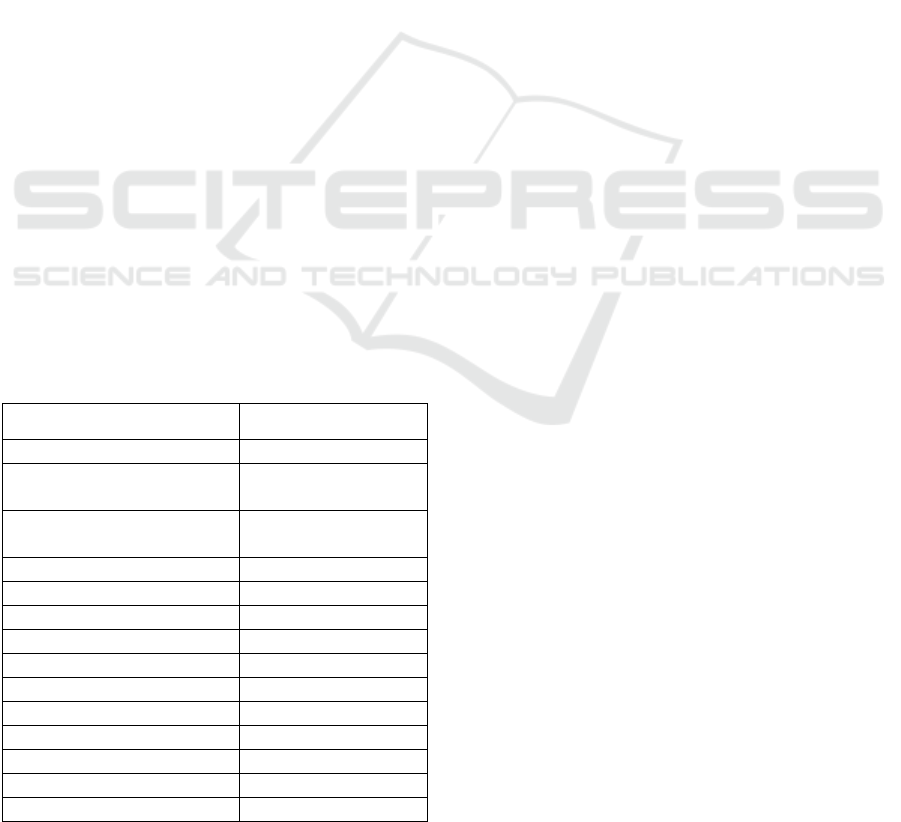
Table 1: Analysis results of native mung bean starch.
Analysis parameters Analysis results
Starch yield 58.44 ± 12.29%
Starch content
(from isolated starch)
88.31 ± 2.68%
Starch content
(from mung bean flour)
29.46 ± 2.30%
Moisture content 6.39% ± 0.29%
Acetyl percentage 0.00%
Degree of substitution 0.00
Amylose 26.79 ± 0.74%
Amylopectin 73.21 ± 0.76%
Solubility 16.48 ± 0.55%
Swelling power 8.83 ± 0.52%
L* value 75.38 ± 0.18
Chroma 7.34 ± 1.18
Resistant starch content 23.96%
Crystallinity 57.94%
Mung bean starch consist of 26,79 ± 0.74%
amylose content and 73.21 ± 0.76% amylopectin
content. According to Kaur et al. (2012), amylose
content of mung bean starch ranged between 29.9 –
33.6%, while amylopectin content reach ±70%. Value
of amylose and amylopectin content can be different,
because of different variety of mung bean plant.
However, same amylose and amylopectin content can
be found even in plants with same variety.
Geographic and environment condition when plants
were planted can affect their amylose and
amylopectin content (Gao et al., 2014).
Amylose and amylopectin content affect
physicochemical properties of starch. High amylose
content (>30%) can increase possibility of starch
retrogradation (Alcazar-Alay & Meireles, 2015).
Moreover, increased amylose content can increase
pasting properties, viscosity, and solubility properties
of starch granules (Colussi et al., 2015). On the other
hand, starch with high amylopectin content (>70%)
has low capacity to absorb water, more resistant to
enzyme digestion and chemical reaction, compared to
starch with high amylose content (Gunaratne &
Corke, 2016).
Starch content obtained from this research is
29.46 ± 2.30%. This result is higher than
Moongngarm et al. (2013) analysis result which is
25.73%. Different sample preparation and isolation
method can affect starch content obtained.
Decorticated mung beans produce more starch yield
and content, compared to whole mung beans (Abdel-
Rahman et al., 2008). Moreover, soaking temperature
(up to 30
o
C) and soaking time (up to 18 hours) of
mung bean in preparation process can increase starch
extraction efficiency. However, soaking in room
temperature for two hours is enough to produce starch
for smaller scale (Usman et al., 2014).
Native starch acetyl percentage and degree of
substitution resulted zero value. Those value was
obtained because there is no acetyl group or glucose
unit bound to acetyl group was found (Colussi et al.,
2105). Resistant starch content obtained was 23.96%.
This value is not much different than analysis result
in Shi et al. (2016) research, which is ranged between
16.1-22.3%. There are some factors affecting starch
digestibility, such as starch structure characteristics
(amylose and amylopectin ratio, gelatinization
degree, retrogradation rate, and formation of amylose
complex), food characteristics, and existence of other
components (Conde-Petit et al., 2001).
2nd SIS 2019 - SEAFAST International Seminar
12

Table 2: Crystallinity percentage of annealed starch.
Annealing
temperature (°C)
Crystallinity percentage
(%)
Native 57.94
40° 63.59
50° 70.73
60° 88.89
3.2 Effect of Annealing Temperature to
Crystallinity Percentage of Mung
Bean Starch
Starch modification with annealing method makes
starch granules structure become more stable,
because of polymer chains re-organization in
crystalline and amorph side that cause increased
crystallinity percentage (Lan et al., 2008). Increasing
temperature between glass transition and starch
gelatinization temperature, can increase hydration
rate and glucan chain mobility (Jayakody & Hoover,
2008). Increased crystallinity percentage of starch can
be detected with XRD in Table 2.
Crystal type of starch can be detected from
diffraction pattern with XRD analysis. XRD analysis
results can be found in Figure 1, and can be concluded
that native starch intensity as well as treated sample
has diffraction angle at 2θ = 15°, 17°, 18°, and 23°
(Colussi et al., 2014). This result showed that mung
bean starch has A-type crystal, in accorandce with
theories that showed peak at 2θ = 23°, but didn’t show
peak at 2θ = 5.6° in A-type crystal graph (Correia et
al., 2012). Phrukwiwattanakul et al. (2014) research
of mung bean starch resulted in similar outcome, with
peak at 2θ = 15°, 17°, 18°, and 23°on the graph. Peak
at same diffraction angle has been found in Colussi et
al. (2014) research too, in rice starch with 20-32%
amylose content. There is no change in starch crystal
type with increased temperature up to 60°C.
Figure 1: XRD analysis results of annealed mung bean
starch.
3.3 Effect of Acetic Acid Anhydride
Concentration and Acetylation
Reaction Time to Mung Bean
Starch
Acetyl percentage is acetyl group amount in every
gram starch sample (wet basis) (Rahim et al., 2017).
Acetyl percentage in annealed and acetylated mung
bean starch can be found in Figure 2. Statistical
results showed that interaction between acetic acid
anhydride concentration and acetylation reaction
time, affect (p<0.05) acetyl percentage of modified
mung bean starch. Difference in acetic acid anhydride
concentration or acetylation reaction time separately,
also affect (p<0.05) acetyl percentage of modified
mung bean starch as well.
According to Figure 2, the highest acetyl
percentage found from acetylated starch with 20%
acetic acid anhydride concentration and 25 minutes
reaction time. Increasing reagent concentration and
reaction time can increase the chance for substitution
group to bind with starch. Increased bonds can
increase acetyl percentage and acetylation reaction
efficiency (Ackar et al., 2015).
Figure 2: Effect of acetylation condition to acetyl
percentage of modified mung bean starch. Different
superscripts indicate significant difference (p<0.05).
Degree of substitution (DS) is average amount of unit
glucose side which bound to substitution group
(acetyl group) (Rahim et al., 2017). DS of annealed
and acetylated mung bean starch can be found on
Figure 3.
Modification of Mung Bean Starch by Annealing Treatment and Acetylation
13

Figure 3: Effect of acetylation condition to degree of
substitution of modified mung bean starch.
Statistical results showed that interaction between
acetic acid anhydride concentration and acetylation
reaction time affect (p<0.05) DS in modified mung
bean starch. Difference in acetic acid anhydride
concentration or acetylation reaction time separately
also affect (p<0.05) DS in modified mung bean
starch. The highest DS found from acetylated starch
with 20% acetic acid anhydride concentration and 25
minutes reaction time. DS is related to acetyl
percentage, more acetyl group found in starch will
also increase the chance of acetyl group to bind with
hydroxyl group in starch (Ackar et al., 2015).
Acetylated starch with 20% acetic acid anhydride
and 15 minutes reaction time, meets FDA
requirement which stated that DS found in starch
can’t exceed 0.2. However, there is no significant
difference (p>0.05) in DS between acetylated starch
with 20% acetic acid anhydride concentration and 15
minutes reaction time, compared to acetylated starch
with 15% acetic acid anhydride and 25 minutes
reaction time. Considering time efficiency and DS
value proximity to FDA requirement, then acetylation
treatment with 20% acetic acid anhydride and 15
minutes reaction time became selected treatment.
Incorporation of acetyl group in starch can be
confirmed with FTIR (Colussi et al., 2014). Result of
FTIR analysis of annealed and acetylated starch with
series of acetic acid anhydride concentration and
acetylation reaction time can be found on Figure 4.
Figure 4: FTIR analysis results of annealed and acetylated
mung bean starch.
Acetylation reaction can subtitute hydroxyl group
in starch molecule to carbonyl contained group. This
occurrence resulted in decreased hydroxyl group
intensity along with increased carbonyl group
intensity. Hydroxyl group intensity in starch can be
found at 3700–3000 cm
-1
. While C=O intensity in
acetyl group can be found at 1700-1500 cm
-1
(Rahim
et al., 2017).
Selected annealing treatment was chosen based on
highest crystallinity percentage. Increased
crystallinity percentage cause decrease in swelling
and solubility, increase in heat stability, and increase
in starch resistance of alpha amylase enzyme (Song et
al., 2011; Siswoyo & Morita, 2010). Therefore 60°C
became selected temperature for annealing, before
acetylation treatment. Additionally, selected
acetylation treatment was based on highest acetyl
percentage and DS that meet FDA requirement. FDA
limit DS of modified starch to maximum 0.2 if the
starch was going to be used as food ingredient (Xu et
al., 2004).
Selected acetylation treatment was combined with
selected annealing treatment, which is 60°C
annealing temperature, 20% acetic acid anhydride
concentration, and 15 minutes acetylation reaction
time. Modified starch with selected treatments was
analyzed with starch content, water content, solubility
properties and swelling power, pasting properties,
XRD profile, resistant starch content, amylose and
amylopectin content, colour test, and starch granule
morphology as parameters.
3.4 Physicohemical Properties of
Selected Starch
Solubility properties and swelling power of mung
bean starch decreased after dual modification.
Swelling power of native starch was decreasing from
18.11% to 11.62%, while solubility properties of
2nd SIS 2019 - SEAFAST International Seminar
14

native starch was decreasing drom 16.48% to 8.83%.
This decreasing value was different significantly
(p<0.05) and can be found in Table 3. Native starch
referred to starch without any treatments, while
selected starch has been annealed and acetylated by
selected treatment. Decreased value of swelling
power and solubility properties can be caused by
annealing treatment, which re-organized starch
crystal to become more compact. Interaction between
amylose and amylopectin from annealing also
decrease hydration of amorph side (Zavareze & Dias,
2011). Moreover, incorporation of acetyl group in
acetylation reaction, makes starch molecule becomes
more hydrophobic (Luo & Shi, 2012).
Basic rheology properties is viscosity, which
affected by temperature, concentration, and shear
stress (Alcazar-Alay & Meireles, 2015). Rheology
properties results can be found in Table 3. Pasting
properties is a term used to explain transformation in
starch after gelatinization. Rapid Visco Analyzer
(RVA) can be used to explain viscosity parameter as
a function to temperature and time.
Starch suspension was given shear forces when
analysis was conducted. Suspension will show peak
viscosity, which started after gelatinization and the
value will increase along with expansion of starch
granule (Alcazar-Alay & Meireles, 2015). Decreased
peak viscosity can be caused by decreased absorption
Table 3: Psycochemical properties of native and selected
starch.
Psycochemical
properties
Native starch
Selected
starch
Solubility (%) 16.48±0.55
a
8.83±0.52
b
Swelling power
(% w/w)
18.11±0.44
a
11.62±0.63
b
Pasting
temperature (°C)
75.8 81.6
Peak viscosity
(cP)
5384 3372
Hot paste
viscosity (cP)
3397 2939
Cold paste
viscosity (cP)
5516 5075
Breakdown (cP) 1987 433
Setback (cP) 132 1703
Resistant starch
(% w/w)
23.96 24.68
Amylose (%) 26.79±0.74
a
24.09±0.72
b
Amylopectin (%) 73.21±0.76
a
75.91±0.68
b
L* value 75.38±0.18
a
75.77±0.10
b
Chroma value 7.34±1.18
a
7.74±0.19
a
capacity of starch (solubility properties) and
decreased starch ability to form a paste (swelling
power) (Marta & Tensiska, 2017).
Breakdown describes a difference value between
peak viscosity and hot paste viscosity. Breakdown
shows starch stability when exposed to heat, while hot
paste viscosity related to heat resistance of starch or
weakness of starch granule chains (Marta & Tensiska,
2017). Decreased value of breakdown will increase
starch stability when heated. This occurrence can be
caused by increased crystallinity and more compact
structure of starch chain when annealing and
acetylation treatment was conducted (Ariyantoro,
A.R. et al., 2018; Mendoza et al., 2016).
Pasting temperature after acetylation treatment
supposed to be lower than native starch, because
incorporation of acetyl group that weakens starch
granule structure, can break the compact structure of
starch granule. However, annealing treatment before
acetylation can increase compactness or crystallinity
of starch structure (Simsek et al., 2012). This
treatment combination can increase pasting
temperature of starch.
Setback is a difference value between cold paste
viscosity and hot paste viscosity. Increasing value of
setback, will increase the chance of retrogradation to
occur (Wang et al., 2015). In cooling period, amylose
leaching will form a three-dimensional gel network.
This gel formation can increase viscosity, which is
cold paste viscosity (Alcazar-Alay & Meireles,
2015). In this research, annealing and acetylation
treatment increase setback value. When annealing
occurred, granule starch texture changed to become
more compact. This occurrence can cause increasing
viscosity when starch cools down and increase
retrogradation rate. Starch with high setback value is
a good gelling agent, which is desirable in certain
food industry (Marta & Tensiska, 2017).
Comparison of XRD analysis results between
native and selected starch can be found in Figure 5.
Mung bean starch crystal type remain unchanged
which is A-type crystal, even after acetylation with
20% acetic acid concentration and 15 minutes
reaction time. Native or treated starch intensity was
found at same diffraction angle which is 2θ = 15°,
17°, 18°, and 23° (Colussi et al., 2014). However,
crystallinity percentage of annealed starch in 60°C
decrease from 88.89% to 87.80% after acetylation.
According to Colussi et al. (2015), decreased
crystallinity percentage after acetylation also
decrease hydrogen bond in starch, resulted in
decreased crystalline structure.
Modification of Mung Bean Starch by Annealing Treatment and Acetylation
15

Figure 5: XRD analysis results of native, annealed (60°C),
and selected starch.
Resistant starch content increased from 23.96% to
24.68% according to Table 3. Heating step in
annealing form more compact structure of starch
granule, because of hydrogen bond between amylose
or amylopectin (Sajilata et al., 2006). Acetylation also
has important role in increasing resistant starch
content. Acetyl group that bind with starch chain, can
hinder active side of α-amylase enzyme that breaks
starch bonds (Sahnoun et al., 2015). α-amylase is an
enzyme that capable of hydrolyze starch randomly at
α-(1→4) D-glucosidic bond, to produce glucose and
oligosaccharide. Starch hydrolysis with α-amylase
can be hindered by acetylation treatment (Chen et al.,
2004).
Colussi et al., (2015) research has used same
acetic acid anhydride concentration, and same
reaction time as selected treatment in this research.
They found no significant difference in resistant
starch between native and acetylated rice starch.
Meanwhile, in Song et al., 2011 research, annealing
before cross-linking treatment can increase resistant
starch content to 31.5%. However, they used 50°C
annealing temperature for 12 hours and cross-linking
reaction time for 4 hours. This founding concluded
that annealing temperature, reagent concentration,
and starch modification reaction timecan affect
hydrolysis rate of starch (Song et al., 2011).
Amylose content of native and modified starch
can be compared in Table 3. Amylose content
decreased from 26.79% to 24.09% after acetylation.
Decreased amylose content resulted in increased
amylopectin content from 73.21% to 75.91%. These
decreasing number was significantly different
(p<0.05).
Acetyl group found in starch granule can hinder
helix structure of amylose to bind with iodine because
of steric hindrance (Gonzales & Perez, 2002).
Another possibility that can cause decreasing
amylose content is depolymerization of amylose
chain. Starch contains polymer chains that can be
broken to monomers by increasing temperature or
degree of substitution (Kapelko-Zeberska et al.,
2017). Decreased amylose content also happened in
acetylated banana starch (Reddy et al., 2014),
acetylated buckwheat starch (Sarkar, 2016), and
acetylated potato starch (Kapelko-Zeberska et al.,
2017).
Figure 6: Physical appearances of mung bean flour, starch
and modified one.
Physical appearance of starch as food ingredient
is important, because it can affect product
acceptability. (Dahiya et al., 2015). Colour difference
between samples can be found in Figure 6.
Decorticated mung bean flour has yellow to brown
colour, while native and annealed mung bean starch
has white colour. However, annealed and acetylated
starch has brighter white colour. L* value and chroma
analysis results can be found in Table 3. Increased L*
value was significantly different (p<0.05), while
increased chroma value was not significantly
different (p>0.05). Acetylation treatment can increase
starch L* value and decrease chroma value. Higher
increase in L* value will make starch appear whiter,
while chroma value describes sample colour purity.
However, chroma value is not really considered to
determine colour characteristic of starch (Bolade &
Oni, 2015). Increasing L* value and decreasing
chroma value is desirable from consumer perspetive
(Ali et al., 2016).
Morphology of starch granule can be observed
using Scanning Electron microscope (SEM). SEM
magnification used in this analysis was 350×, 750×,
1500× and 3000×. Magnification 3000× was used to
observe starch granule surface and shape more
clearly. While magnification 350×, 750×, and 1500×
was used to ensure starch granule condition in 3000×
was found on another magnification too. Mung bean
starch has granule size between 7.65 - 33.15 µm
(Abdel-Rahman et al., 2008). Mung bean granule
starch can be found in Figure 7.
2nd SIS 2019 - SEAFAST International Seminar
16
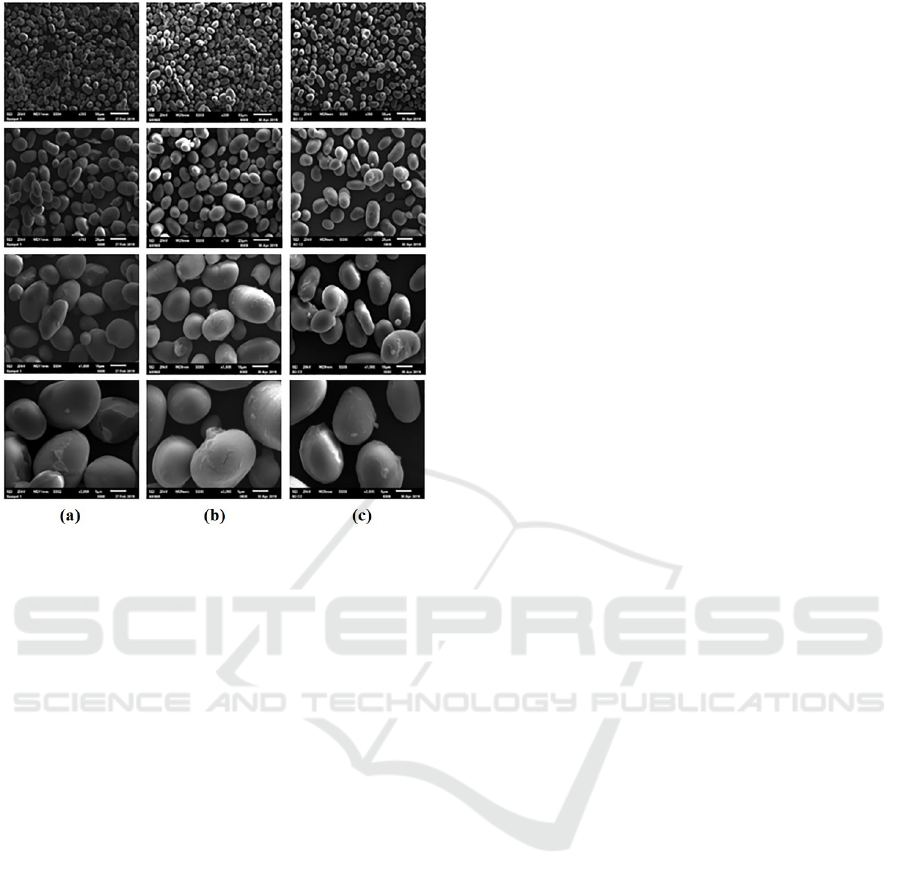
Figure 7: SEM analysis results of native starch (a), annealed
starch (b), and annealed-acetylated starch (c).
Magnifications used from top to bottom figure were 350×,
750×, 1500×, and 3000×.
Mung bean granule shape remains unchanged
when observed with 3000× magnification. This
occurrence also found in 350×, 750× and 1500×
magnification. Bigger mung bean starch granule has
oval shape, while smaller one has round shape (Liu &
Shen, 2007).
Several native and modified starch granules have
uneven surface, but the particle structure is still intact.
Uneven surface can be observed clearly in 3000×
magnification, but there’s no deformed starch granule
found even in 350×, 750× and 1500× magnification.
Uneven starch granules can be caused by drying
temperature starts from ± 40°C (Lewicka et al., 2015).
Heating process can break hydrogen bonding
between starch polymer chains, resulting weaker
granule structure and rough granule starch surface
(Nadiah et al., 2015). Deformed starch granule has
higher capacity to absorb water, making it more
susceptible to enzyme hydrolysis and high
temperature (Ali et al., 2014). Therefore, intact
granule starch shape is desirable, especially if added
into food product that used high temperature in the
process.
4 CONCLUSIONS
Increasing annealing temperature up to 60°C can
increase crystallinity percentage. Other than that,
increasing acetic acid anhydride concentration and
acetylation reaction time, can cause increased starch
acetyl percentage and degree of substitution. Selected
treatment for starch analysis was annealing with 60°C
temperature, and acetylation with 20% acetic acid
anhydride concentration for 15 minutes reaction time.
Starch produced from combination of annealing and
acetylation with selected treatment, has higher
resistance and stability when heated, along with higher
resistant starch content compared to native starch.
Combination of annealing and acetylation
treatment to mung bean starch, caused decreased
amylose content and increased amylopectin content.
Solubility properties and swelling power value
decreased, correspond with decreased peak viscosity
in pasting properties analysis. Decreased peak
viscosity was followed by decreased breakdown
value, increased pasting temperature, and increased
setback value. Modified starch has brighter colour,
and its granule shape remains unchanged compared to
native starch. Starch with these physicochemical
characteristics were suitable to be used as food
ingredient in cookies or cereal industries.
REFERENCES
Abdel-Rahman E.A., El-Fishawy F.A., El-Geddawy M.A.,
Kurz, T., El-Rify M.N. (2008): Isolation and Physico-
chemical Characterization of Mung Bean Starches.
International Journal of Food Engineering, 4: 1-12.
Abeysundara A., Navaratne S., Wickramasinghe I.,
Ekanayake D. (2015): Determination of Changes of
Amylose and Amylopectin Content of Paddy during
Early Storage. International Journal of Science and
Research, 6: 2094-2097.
Ackar D., Babic J., Jozinovic A., Milicevic B., Jokic S.,
Milicevic R., Rajic M., Subaric D. (2015): Starch
Modification by Organic Acids and Their Derivatives:
A Review. Molecules, 20: 19554-19570.
Alcazar-Alay S.C., Meireles M.A. (2015):
Physicochemical Properties, Modifications and
Applications of Starches from Different Botanical
Sources. Food Science and Technology, 35: 215-236.
Ali A., Wani T.A., Wani I.A., Masoodi F.A. (2016).
Comparative Study of The Physico-Chemical
Properties of Rice and Corn Starches Grown in Indian
Temperate Climate. Journal of the Saudi Society of
Agricultural Sciences, 15: 75-82.
Ali R., Khan M.S., Sayeed S.A., Ahmed R., Saeed S.M.G.
dan Mobin, L. (2014): Relationship of Damaged Starch
with Some Physicochemical Parameters in Assessment
Modification of Mung Bean Starch by Annealing Treatment and Acetylation
17

of Wheat Flour Quality. Pakistan Journal of Botany, 46:
2217-2225.
AOAC. (2005): Official Methods of Analysis of the
Association of Official Analytical Chemists, 18th ed.
USA: AOAC, Inc.
Ariyantoro A.R., Katsuno N., Nishizu T. (2018): Effects of
Dual Modification with Succinylation and Annealing
on Physicochemical, Thermal and Morphological
Properties of Corn Starch. Foods, 7: 133-145.
Bolade M.K., Oni O.J. (2015): Influence of Acetylation on
The Physicochemical Properties of Composited
Starches from Sweet Potato (Ipomoea Batatas L.) And
Water Yam (Dioscorea alata L.). African Journal of
Biotechnology, 14: 3340-3349.
Chen Z., Schols, H.A., Voragen, A.G.J. (2004): Differently
Sized Granules from Acetylated Potato and Sweet
Potato Starches Differ in The Acetyl Substitution
Pattern of Their Amylose Populations. Carbohydrate
Polymers, 56: 219-226.
Colussi R., Halal S.L., Pinto V.Z., Bartz J., Gutkoski L.C.,
Zavareze E.R., Dias A.R. (2015): Acetylation of Rice
Starch in an Aqueous Medium for Use in Food. Food
Science and Technology, 62: 1076-1082.
Colussi R., Pinto V.Z., El-Halal S.L.M., Vanier N.L.,
Villanova F.A., E-Silva R.M., Zavareze E.R., Dias
A.R.G. (2014): Structural, Morphological, and
Physicochemical Properties of Acetylated High-,
Medium-, and Low-Amylose Rice Starches.
Carbohydrate Polymers, 103: 405-413.
Conde-Petit B., Nuessli J., Arrigoni E., Escher F., Amado
R. (2001): Perspectives of Starch in Food Science.
Chimia, 55: 201-205.
Dahiya P.K., Linnemann A.R., Van Boekel M.A.J.S.,
Khetarpaul N., Grewal R.B., Nout, M.J.R. (2015):
Mung Bean: Technological and Nutritional Potential.
Critical Reviewsin Food Science and Nutrition, 55:
670-688.
Ezeigbo O.R., Ukabi C.F., Ike-Amadi C.A., Ekaiko, M.U.
(2015): Determination of Starch and Cyanide Contents
of Different Species of Fresh Cassava Tuber in Abia
State, Nigeria. British Biotechnology Journal, 6: 10-15.
Ganesan K., Xu B. (2018): A Critical Review on
Phytochemical Profile and Health Promoting Effects of
Mung Bean (Vigna radiata). Food Science and Human
Wellness, 7: 11-33.
Gao H., Cai J., Han W., Huai H., Chen Y., Wei C. (2014):
Comparison of Starches Isolated from Three Different
Trapa Species. Food Hydrocolloids, 37: 174-181.
Gonzales Z., Perez E. (2002): Effect of Acetylation on
Some Properties of Rice Starch. Starch/Stärke, 54: 148-
154.
Gunaratne A., Corke H. (2016): Starch Analysis of Quality.
Reference module in Food Science, 3: 202-212.
Jayakody L., Hoover R. (2008): Effect of Annealing on The
Molecular Structure and Physicochemical Properties of
Starches from Different Botanical Origins: A Review.
Carbohydrate Polymers, 74: 691-703.
Kapelko-Zeberska M., Zieba T., Spychaj R., Gryszkin A.
(2017): Selected Rheological Properties of RS3/4 Type
Resistant Starch. Polish Journal of Food and Nutrition
Sciences, 67: 293-299.
Kaur B., Ariffin F., Bhat R., Karim A.A. (2012): Progress
in Starch Modification in The Last Decade. Food
Hydrocolloids, 26: 398-404.
Kementerian Pertanian. (2018): Statistik Konsumsi Pangan
Tahun 2018. Jakarta: Pusat Data and Sistem Informasi
Pertanian.
Lan H., Hoover R., Jayakody L., Liu Q., Donner E., Baga
M., Asare E.K., Hucl P., Chibbar R.N. (2008): Impact
of Annealing on The Molecular Structure and
Physicochemical Properties of Normal, Waxy and High
Amylose Bread Wheat Starches. Food Chemistry, 111:
663-675.
Lewicka K., Siemion P., Kurcok P. (2015): Chemical
Modifications of Starch: Microwave Effect.
International Journal of Polymer Science, 1-10.
Liu W., Shen Q. (2007): Studies on The Physicochemical
Properties of Mung Bean Starch from Sour Liquid
Processing and Centrifugation. Journal of Food
Engineering, 79: 358-363.
Lopez D.V., Zaritzky N.E., García, M.A. (2010):
Physicochemical Characterization of Chemically
Modified Corn Starches Related to Rheological
Behavior, Retrogradation and Film Forming Capacity.
Journal of Food Engineering, 100: 160-168.
Luo Z.G., Shi Y.C. (2012): Preparation of Acetylated
Waxy, Normal, and High-Amylose Maize Starches
with Intermediate Degrees of Substitution in Aqueous
Solution and Their Properties. Journal of Agricultural
and Food Chemistry, 60: 9468-9475.
Marta H., Tensiska. (2017): Functional and Amylographic
Properties of Physically-Modified Sweet Potato Starch.
2nd International Conference on Sustainable
Agriculture and Food Security: A Comprehensive
Approach, 689–700.
Mendoza J.S., RuyDiaz J.H., Quintero A.F. (2016): Effect
of The Acetylation Process on Native Starches of Yam
(Dioscorea spp.). Revista Facultad Nacional de
Agronomia, 69: 7997-8006.
Moongngarm A. (2013): Chemical Compositions and
Resistant Starch Content in Starchy Foods. American
Journal of Agricultural and Biological Sciences, 8: 107-
113.
Nadiah N.I., Uthumporn U., Syahariza Z.A. (2015): Effect
of Microwave Heating on Potato and Tapioca Starches
in Water Suspension. International Journal on
Advanced Science Engineering Information
Technology, 5: 264-271.
Nadir A.S., Helmy I.M.F., Nahed M.A., Wafaa M.M.A.,
Ramadan M.T. (2015): Modification of Potato Starch
by Some Different Physical Methods and Utilization in
Cookies Production. International Journal of Current
Microbiology and Applied Sciences, 4: 556-569.
Phrukwiwattanakul P., Wichienchotand S., Sirivongpaisal
P. (2014): Comparative Studies on Physico-Chemical
Properties of Starches from Jackfruit Seed and Mung
Bean. International Journal of Food Properties, 17:
1965-1976.
2nd SIS 2019 - SEAFAST International Seminar
18

Rahim A., Kadir S., Jusman (2017): The Influence Degree
of Substitution on The Physicochemical Properties of
Acetylated Arenga Starches. International Food
Research Journal, 24: 102-107.
Ratnayake W. S., Wassinger A.B., Jackson D.S. (2007):
Extraction and Characterization of Starch from
Alkaline Cooked Corn Masa. Cereal Chemistry, 84:
415-422.
Reddy C.K., Haripriya S., Suriya M. (2014): Effect of
Acetylation on Morphology, Pasting and Functional
Properties of Starch from Banana (MUSA AAB).
Indian Journal of Scientific Research and Technology,
2: 31-36.
Sahnoun M., Ismail N., Kammoun R. (2016):
Enzymatically Hydrolysed, Acetylated and Dually
Modified Corn Starch: Physico-Chemical, Rheological
and Nutritional Properties and Effects on Cake Quality.
Journal of Food Science and Technology, 53: 481-490.
Sajilata M.G., Singhal R.S., Kulkarni, P.R. (2006):
Resistant Starch: A Review. Comprehensive Reviews
in Food Science and Food Safety, 5: 1-17.
Sarkar S. (2016): Influence of Acetylation and Heat-
Moisture Treatment on Physio-Chemical, Pasting and
Morphological Properties of Buckwheat (Fagopyrum
esculentum) Starch. Asian Journal of Dairy and Food
Research, 35: 298-303.
Simsek S., Ovando-Martinez M., Whitney K., Bello-Perez
L.A. (2012). Effect of Acetylation, Oxidation and
Annealing on Physicochemical Properties of Bean
Starch. Food Chemistry, 134: 1796-1803.
Siswoyo T.A., Morita N. (2010): Influence of Annealing on
Gelatinization Properties, Retrogradation and
Susceptibility of Breadfruit Starch (Artocarpus
Communis). International Journal of Food Properties,
13: 553–561.
Song J.Y., Park J.H., Shin M. (2011): The Effects of
Annealing and Acid Hydrolysis on Resistant Starch
Level and The Properties of Cross-Linked RS4 Rice
Starch. Starch/Stärke, 63: 147-153.
Usman M., Ishfaq M.T., Malik S.R., Ishfaq B., Iqbal M.
(2014). Effects of Temperature, pH and Steeping Time
on the Extraction of Starch from Pakistani Rice
Muhammad. International Journal of Scientific &
Engineering Research, 5: 857-892.
Wang S., Li C., Copeland L., Niu C., Wang S. (2015):
Starch Retrogradation: A Comprehensive Review.
Comprehensive Reviews in Food Science and Food
Safety, 14: 568-585.
Xu Y., Miladinov V., Hanna M.A. (2004): Synthesis and
Characterization of Starch Acetates with High
Substitution. Cereal Chemistry, 81: 735-740.
Zaman A.S., Seruji A.Z., Sarbini S.R. (2015): Effect of
Acetylation on Physicochemical Properties and
Resistant Starch Content of Metroxylon sagu Starch.
International Conference on Food Nutrition, Chemical
and Environmental Engineering. Kuala Lumpur,
Malaysia.
Zavareze E.R., Dias, A.R.G. (2011): Impact of Heat-
Moisture Treatment and Annealing in Starches: A
Review. Carbohydrate Polymers, 83: 317-328.
Modification of Mung Bean Starch by Annealing Treatment and Acetylation
19
