
Antibacterial Activity and RP-HPLC Characteristic of Lysozyme
from Local Chicken Egg White after Modification Treatments
Syahrizal Nasution
1
, Didah Nur Faridah
2
, Eni Kusumaningtyas
3
, Zakiah Wulandari
4
and Harsi Dewantari Kusumaningrum
2
1
Study Program of Food Science, Graduate School, IPB University, Bogor 16680, Indonesia
2
Department of Food Science and Technology, Faculty of Agricultural Technology, IPB University, Bogor 16680, Indonesia
3
Indonesian Research Center for Veterinary Sciences, Bogor 16114, Indonesia
4
Department of Animal Production and Technology, Faculty of Animal Science, IPB University, Bogor 16680, Indonesia
Keywords: Antibacterial, Food Safety, Lysozyme, MIC, RP-HPLC.
Abstract: Lysozyme is a globular protein and is a hydrolase enzyme. Lysozyme isolated from chicken egg whites can
damage the membrane of bacteria hence it can be used as an antibacterial. The purpose of the study was to
modify, to measure antibacterial activity, and to characterize the lysozyme from local chicken egg whites.
The isolate of lysozyme was modified by heat modification treatments at 60°C, 75°C, and 90°C in pH seven
buffer solution. The antibacterial activity of lysozyme was measured by the micro-dilution method. The
isolate and the modified lysozyme were characterized by reversed phase-high performance liquid
chromatography (RP-HPLC). The results showed that heat modification treatments decreased the minimum
inhibitory concentration of lysozymes to Gram-positive bacteria up to 2-fold from 6 mg/mL to 3 mg/mL. Heat
modification also improved antibacterial spectrum of lysozyme against Gram-negative bacteria. The RP-
HPLC chromatograms of modified lysozyme showed the peak of lysozyme at 43.59 ± 0.09 minutes retained
time had decreased. It was suggested that any concentration of lysozyme was retained on the different retained
times doe to the modification of lysozyme. The characteristic of RP-HPLC had explained the reason for
increasing the antibacterial activities of lysozyme after modification treatments.
1 INTRODUCTION
Lysozyme is a hydrolase enzyme in the form of
antibacterial protein that can be isolated from the
chicken egg white (Gyawali and Ibrahim 2014). The
chicken egg white has excellent potential as a source
of lysozyme because it contains about 2500-3000
µg/mL of lysozyme and can be obtained from some
of the industrial waste (Lesnierowski and Kijowski
2007). Lysozyme can be used directly, combined
with antimicrobials on food, also added to edible
packaging so that it can improve food safety
(Kijowski et al. 2002; Herath et al. 2015).
The food industry had been using lysozyme
because it has a stable primary structure at heat and
low pH treatments. The mechanism of antibacterial
activity of lysozyme occurs through peptidoglycan
destruction on bacterial cell wall membrane by active
site of the lysozyme, which breaks the glycosidic
bond between N-acetylglucosamine and N-
acetylmuramic acid (Susanto et al. 2013).
The antibacterial activity of isolate lysozyme in
native form is still limited to Gram-positive bacteria,
whereas Gram-negative bacteria could be food
contaminants (Lesnierowski et al. 2001). Therefore,
it is necessary to modify lysozyme to improve its
antibacterial activity against Gram-negative bacteria
that are more resistant because the bacterial cell wall
is coated by lipopolysaccharide (Cegielska-
Radziejewska et al. 2008; Susanto et al. 2013). One
of simple methods that used to modify lysozyme was
heat treatments (Nasution et al. 2018). Besides, after
heat modification treatments, the characterization of
lysozyme by RP-HPLC used to explained the
changed of antibacterial activity of modified
lysozyme that have never been reported.
2 MATERIALS AND METHODS
2.1 Lysozyme
Respectively, a local and commercial isolate of
lysozyme were obtained from Sentul chicken eggs at
84
Nasution, S., Faridah, D., Kusumaningtyas, E., Wulandari, Z. and Kusumaningrum, H.
Antibacterial Activity and RP-HPLC Characteristic of Lysozyme from Local Chicken Egg White after Modification Treatments.
DOI: 10.5220/0009979300002833
In Proceedings of the 2nd SEAFAST International Seminar (2nd SIS 2019) - Facing Future Challenges: Sustainable Food Safety, Quality and Nutrition, pages 84-88
ISBN: 978-989-758-466-4
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

the Research Institute for Animal Production
(Balitnak, Ciawi-Bogor) and commercial chicken
eggs wholesaler (Kurnia Jaya, Dramaga-Bogor).
Both of lysozyme was isolated with Amberlite
FPC3500 resin. The standard lysozyme (L-6876,
from chicken egg white, 47,000 unit/mg solid) was
obtained from Sigma-Aldrich. The lysozyme sample
was prepared in 10 mL of 10 mM potassium
phosphate buffer pH seven.
2.2 Modification of Lysozyme
The lysozyme was modified by heat treatment
(Carrillo et al. 2018). As much as 6 mg/mL of each
isolate lysozyme in 10 mL of 10 mM potassium
phosphate buffer pH seven was heated in a water bath
in each heat temperature of treatments (60°C, 75°C,
and 90°C) for 20 minutes. The solution was placed in
the refrigerator (4°C) after having heated.
2.3 Measurement of Antibacterial
Activity
Antibacterial activity of lysozyme was measured
based on the micro-dilution method concerning on
Clinical and Laboratory Standards Institute (CLSI)
(Balouiri et al. 2016). As much as 50 μL MHB was
put into each 96-well microplate. Then, 50 μL from 6
mg/mL of each isolate lysozyme or modified as initial
lysozyme was added. Afterward, the initial lysozyme
was diluted four times to the Mueller Hinton Broth
(MHB) until it reached 0.38 mg/mL of lysozyme
dilution concentration. Before having incubated, it
was added 50 μL tested bacteria culture having
prepared to each well. The bacteria stock was made
in sideways MHA media and incubated at 37°C as
long as 24 hours in MHB when it will be utilized.
Control as much as 150 μL used was put in separated
well as MHB media, tested bacteria, and standard
lysozyme solution. Then, a 96-well microplate in
covered condition was incubated (37°C) for 24 hours
before the MIC of lysozyme being evaluated.
The MIC lysozyme was considered as a
concentration that triggers the viewless growth of
bacteria in well after growing as long as 24 hours (not
turbid and no precipitate). Determining MIC, it was
confirmed with a microplate reader at 655 nm. The
minimum bactericidal concentration (MBC) was
obtained by scratching the MIC result to the MHA in
the dish. The not turbid mixture in the well was taken
and scratched to MHA in the dish, then incubated
(37°C) for 24 hours. MBC of lysozyme was
considered as a concentration having no growth of
bacteria in media of each scratch.
2.4 The RP-HPLC Characterization
The lysozyme was characterized by RP-HPLC
(Carrillo et al. 2014 and Kusumaningtyas et al. 2015).
The sample of the isolate or the modified of lysozyme
was injected as much as ten μL utilizing Colom C-18,
possessing reverse-phase high-performance liquid
chromatography (RP-HPLC) system in 215 nm
wavelength for 50 minutes (1 mL/minutes speed at
29.3°C). Before being injected, the sample was
refined with nylon membrane 0.45 μm. Dissolver A
contains 0.37% trifluoroacetic acid in double distilled
water, while dissolver B consists of 0.27 %
trifluoroacetic acid in acetonitrile grade HPLC. The
HPLC system was balanced with 95% solution A (5
minutes), followed by gradient 5-45% of solution A
(5 minutes). The concentration of isolate lysozyme
was calculated by comparing the peak of local isolate
lysozyme to the peak of standard lysozyme.
2.5 Data Analysis
The data was portrayed as the average result ±
standard deviation and also analyzed with Microsoft
Excel, XLSTAT, and SPSS. The result of the analysis
was used to compare the significance of the data.
3 RESULTS AND DISCUSIONS
3.1 Antibacterial Activity of Lysozyme
The research on improving the antibacterial activity
of lysozyme by modification of heat on 60°C, 75°C,
and 90°C through measurements of MIC value of
lysozyme from the local chicken have never been
published ultimately. The MIC value was the
minimum inhibitory concentration of lysozyme
against bacteria or a concentration that does not show
bacterial growth in the well (not cloudy and no
deposits) after 24 hours of incubation. The results
showed that the MIC values of isolate lysozyme were
the same as standard lysozyme in all four bacteria. All
of modified lysozyme from local chicken could
inhibit all bacteria from Gram-positive and Gram-
negative but have slightly different in MIC values.
The MIC values of isolate and modified lysozyme
from local chicken were 3 mg/mL and 6 mg/mL,
which were the concentrations of 50% and 100% of
the solution of lysozyme tested (Table 1).
Antibacterial Activity and RP-HPLC Characteristic of Lysozyme from Local Chicken Egg White after Modification Treatments
85
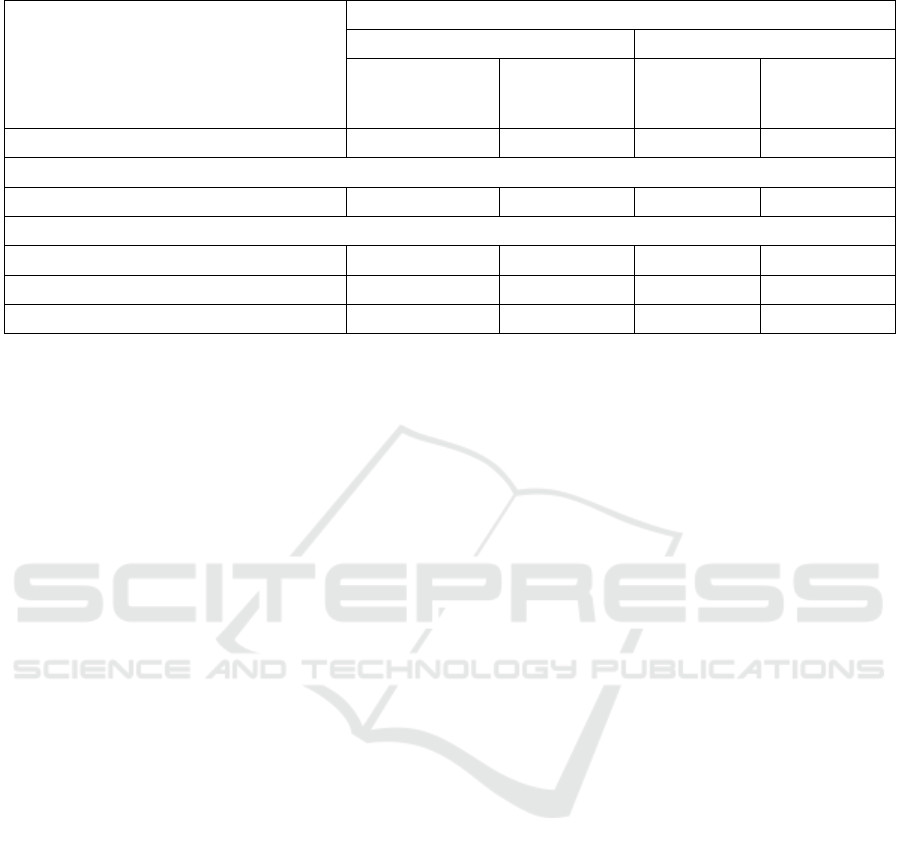
Table 1: The minimum inhibitory concentration of lysozyme towards Gram-positive and Gram-negative bacteria.
Lysozyme types and treatments
modifications
The minimum inhibitory concentration of lysozyme (mg/mL)
Gram-positive bacteria Gram-negative bacteria
Staphylococcus
aureus ATCC
25923
Bacillus
cereus ATCC
10876
Escherichia
coli ATCC
25922
Salmonella
Typhimurium
ATCC 14028
Standard lysozyme (7) 6
a
6
a
˃ 6
a
˃ 6
a
Before modification treatments
Isolate lysozyme (native) (7) 6
a
6
a
˃ 6
a
˃ 6
a
After heat modification treatments
Heat-modified lysozyme 60°C (7) 6
a
6
a
6
b
6
a
Heat-modified lysozyme 75°C (7) 3
b
6
a
6
b
6
a
Heat-modified lysozyme 90°C (7) 3
b
3
b
6
b
6
a
Numbers followed by the same letter were not significantly different (p>0.05) on each bacteria
The improvement of antibacterial activity of
lysozyme from local chicken egg white by heat
modification at pH seven towards Gram-positive and
Gram-negative bacteria can be seen in Table 1. The
heat-modified lysozyme at 75°C and 90°C, when
compared to standard and isolate lysozyme, can
decrease MIC of lysozyme from local chicken egg
white against S. aureus ATCC 25923, and lysozyme
modified by 90°C also decreased MIC of lysozyme
against B. cereus ATCC 10876, whereas at all heat-
modified temperatures, also decreased MIC of
lysozyme against E. coli ATCC 25922 and S.
Typhimurium ATCC 14028.
The MIC values of lysozyme in Gram-negative
bacteria were still higher than in Gram-positive
bacteria. This condition showed that the antibacterial
activity of isolate and modified lysozyme was higher
in Gram-positive bacteria than Gram-negative
bacteria, which were more resistant due to the
presence of lipopolysaccharide as protection
(Lesnierowski et al. 2001). The results f the
improvement of antibacterial activity were in
accordance with other research (Carrillo et al. 2014),
which showed an increasing in the antibacterial
spectrum.
The difference of MIC values at each heat
modification at 60°C, 75°C, and 90°C for the two
Gram-positive bacteria tested was suggested to be
due to changes different in the lysozyme sample. The
improvement of antibacterial activity by heat
modification occurs because it was triggered by
changes in the conformation of lysozyme molecules
from the monomers form to dimers or polymers
(Lesnierowski et al. 2001; 2004; Carrillo et al. 2014;
Vilcacundo et al. 2018). The research conducted by
Cegielska-Radziejewska et al. (2008) reported that
dimer forms in heat-modified lysozyme make the
lysozyme easily attach to lipopolysaccharide in the
bacterial membrane of Gram-negative cell due to
changes in the composition and hence increased the
hydrophobicity of the outside part of lysozyme
molecule. The lysozyme attaches membrane will
disrupt the electrochemical process and the stability
of the lipid bilayer so that it can form holes in the
bacterial membrane. Heat modification can increase
the antibacterial activity against Gram-negative
bacteria through opening the folds of the lysozyme
and expanding the lysozyme bonds in the membrane.
This condition was explained by Ibrahim et al.
1996 that the globular structure of lysozyme has been
dominanted by hydrophobic amino acids inside the
molecule and hydrophilic amino acids outside the
molecule. Heat modification causes the denaturation
process of the globular structure of lysozyme doe to
the two cysteine (Cys) as lysozyme bridges were cut
off so that the hydrophobicity of lysozyme increases.
Breaking disulfide (Cys64-Cys80 bond) and (Cys76-
Cys94 bond) by heat modification causes the
tryptophan 62, 63, and 108 (hydrophobic) in the
globular structure of lysozyme to be exposed and will
come to outside and then contact with bacterial
membranes. The denaturation process causes some or
all of the globular structure of lysozyme to change the
tertiary and secondary lysozyme structures into
primary structures. Heat modification will cause the
hydrophobicity of the outside part of the lysozyme
molecule, and the attach ability of lysozyme to the
bacterial membrane increased. This condition was the
reason for the improvement of the antibacterial
activity of lysozyme against Gram-negative bacteria
and still maintains against Gram-positive bacteria.
2nd SIS 2019 - SEAFAST International Seminar
86

3.2 RP-HPLC Characteristic of
Lysozyme
The characterization of isolate and modified
lysozyme by RP-HPLC method aims to see the
effect of heat modification treatments on the
lysozyme structure from local chicken egg white
based on the tertiary structure of lysozyme as
globular protein and its relation to the antibacterial
activity of isolate and modified of lysozyme. The
results showed that the lysozyme was detected on
43.59 ± 0.09 minutes retained time close to
researched by Carrillo et al. (2016) that detected the
lysozyme on 38 minutes retained time.
Table 2: The concentration of lysozyme.
Lysozyme types
Concentration
(mg/g)
Before heat modification treatments
Isolated lysozyme 41.07 ± 4.44
a
After heat modification treatments
Heat-modified lysozyme 60°C 38.87 ± 4.20
ab
Heat-modified lysozyme 75°C 8.53 ± 0.92
c
Heat-modified lysozyme 90°C 0.99 ± 0.11
c
Numbers followed by the same letter were not significantly
different (p>0.05) among the isolate and modified
lysozyme.
The modification decreased the concentration of
lysozyme as globular proteins detected in lysozyme
from local chicken egg white, which was calculated
based on comparison with standard lysozyme
(Table 2). The reduction of isolated lysozyme
concentration detected at 215 nm wavelength
indicates that isolated lysozyme from local chicken
egg white was not as same as the concentration of
standard lysozyme. The increasing of heat
temperature makes lowering the concentration of
lysozyme. The highest concentration of isolate
lysozyme was found in lysozyme without heat
modification treatments compare to heat-modified
lysozymes.
The reduction of concentration was suggested
because by the structure of lysozyme from local
chicken egg white underwent reactions in running
process through the RP-HPLC column such as
denaturation due to the modifications that caused
changes the lysozyme so that only some
concentration of lysozyme was detected at 43.59 ±
0.09 minutes retained time as same with the
standard lysozyme. Some concentration of
lysozyme from local chicken egg white was thought
to undergo dimerization at an oven temperature of
29.3°C during running RP-HPLC because dimer
conformation can also be formed between 20°C to
30°C as the reversible character of lysozyme at
(Cegielska-Radziejewska et al. 2008).
Based on Table 2, although the detected
lysozyme concentration was reduced, it is evident
in the lysozyme sample that there were other
proteins of modified lysozyme, which were the
reason for the increasing of antibacterial activity of
lysozyme after modification. That protein might be
detected at other retention times. Research
Cosentino et al. (2015) showed that the
concentration of lysozyme in the type of milk
detected by the HPLC method still had the same
concentration after pasteurization (63° C) for 30
minutes. This condition showed that although the
modification of lysozyme heat in this study caused
the lysozyme concentration to decrease, the
concentration of lysozyme contained in the food
system can survive after heat treatment.
Figure 1: The profile of RP-HPLC chromatograms of lysozyme.
Antibacterial Activity and RP-HPLC Characteristic of Lysozyme from Local Chicken Egg White after Modification Treatments
87

The heat modification to the lysozyme showed
that decreasing the peak of lysozyme on (Figure 2).
The increasing of heat temperature makes the peak of
lysozyme to be lower. This condition showed that the
structure of lysozyme was changed that changing the
composition of amino acid of lysozyme from some of
the concentrations of lysozymes. So in the highest
heat temperature of modification treatments made the
peak of lysozyme was the lowest among others.
Besides, column C-18 in RP-HPLC will attach the
modified lysozyme based on the hydrophobicity of
modified lysozymes. This condition showed that the
heat modification treatments can decrease the
concentration of lysozyme from the local chicken egg
white and change the hydrophobicity of modified
lysozymes.
4 CONCLUSIONS
The lysozyme from local chicken egg white was
modified by heat modification treatments. The heat
modification treatments improved antibacterial
activity of lysozyme by decreasing the MIC value.
Otherwise, the isolate and the modified lysozyme
were characterized by RP-HPLC, and the RP-HPLC
chromatograms explaining the reason for the
increasing of antibacterial activity of lysozyme after
modification treatments.
ACKNOWLEDGEMENTS
This work was funded by Direktorat Riset and
Pengabdian Masyarakat, Ditjen Penguatan Riset,
dan Pengembangan, Kementerian Riset, Teknologi
and Pendidikan Tinggi (142/SP2H/LT/DRPM/
2018). Authors thank the Indonesian Research
Institute for Animal Production (Balitnak Ciawi-
Bogor) that providing Sentul chicken eggs.
REFERENCES
Balouiri M, Sadiki M, Ibnsouda SK, 2016. Methods for in
vitro evaluating antimicrobial activity. Journal of
Pharmaceutical Analysis. 6:71-79.
Carrillo W, Lucio, A, Gaibor J, Morales D, Vasquez G,
2018. Isolation of Antibacterial Hydrolysates from hen
egg white lysozyme and identification of antibacterial
peptides. J Med Food. 1-11.
Carrillo W, Tubon J, dan Vilcacundo R. 2016. Isolation of
hen egg white lysozyme by cation exchange
chromatography, analysis of its digestibility and
evaluation of the inhibition lipid peroxidation in the
zebrafish model. Asian J Pharm Clin Res. 9(3):345-
349.
Carrillo W, García-Ruiz A, Recio I, Moreno-Arribas MV,
2014. Antibacterial activity of hen egg white lysozyme
modified by heat and enzymatic treatments against
oenological lactic acid bacteria and acetic acid bacteria.
J Food Prot. 77(10):1732-1739.
Cegielska-Radziejewska R, Lesnierowski G, Kijowski, J,
2008. Properties and aplication of egg white lysozyme
and its modified preparations-a review. Pol. J. Food
Nutr Sci. 58(1):5-10.
Cosentino C, Labella C, Elshafie HS, Camele I, Musto M,
Paolin R, D’adamo C, Freschi P, 2015. Effects of
different heat treatments on lysozyme quantity and
antimicrobial activity of jenny milk. J Dairy Sci.
99:5173-5179.
Gyawali R, Ibrahim SA, 2014. Natural products as
antimicrobial agents. Food Control. 46:412-429.
Herath AHMEI, Priyanath JJM, Ahn DU, Abeyrathne
EDNS, 2015. Use of lysozyme from chicken egg white
as a nitrite replacer in an Italian-type chicken sausage.
FFHD. 5(9):320-330.
Ibrahim HR, Higashiguchi S, Keats M, Juneja F,
Yamamoto T, Sugimoto Y, Aoki T, 1996. Partially
unfolded lysozyme of neutral pH agglutinates and kill
Gram-negative and Gram-positive bacteria through
membrane damage mechanism. J Agric Food Chem.
44:3799–3806.
Kijowski J, Marciszewska C, Cegielska-Radziejewska R,
2002. Quality and microbiological stability of chilled
chicken legs treated with lysozyme. J Food and Nut Sci.
11(52):47-54.
Kusumaningtyas E, Suhartono MT, Widiastuti R,
Kusumaningrum HD, 2015. Antimicrobial and
antioxidative activities of peptides from goat milk
hydrolyzed with various protease. JITV. 20(3):175-
183.
Lesnierowski G, dan Kijowski J, 2007. Lysozyme.
Bioactive egg compounds. Springer-Verlag Berlin
Heidelberg.
Lesnierowski G, Cegielska-Radziejewska R, Kijowski J,
2004. Thermally and chemical-thermally modified
lysozyme and its bacteriostatic activity. WPSJ. 60:303-
310.
Lesnierowski G, Cegielska-Radziejewska R, Kijowski J,
2001. Antibacterial activity of thermally modified
lysozyme. Elec J of Pol Agr Universities. 4 (2):1-9.
Nasution S, Kusumaningtyas E, Faridah DN,
Kusumaningrum HD. 2018. Lysozyme from chicken
egg white as an antibacterial agent. Wartazoa.
28(4):175-188.
Susanto E, Rosyidi D, Radiati LE, Manab A, 2013.
Improved antibacterial spectrum of hen egg white
lysozyme with thermal modified. IJRT. 2:589-593.
Vilcacundo R, Mendez P, Reyes W, Romero H, Pinto A,
Carrillo W, 2018. Antibacterial activity of hen egg
white lysozyme denatured by thermal and chemical
treatments. Sci Pharm 86(48):1-17.
2nd SIS 2019 - SEAFAST International Seminar
88
