
Screening of Toxigenic Aspergillus flavus Strains and
Aflatoxin Content from Agricultural Commodities in Indonesia
Anidah
1,2
, Winiati P. Rahayu
1,3
and Siti Nurjanah
1,3
1
Department of Food Science and Technology, IPB University, Darmaga Campus Bogor 16680, Indonesia
2
SEAMEO BIOTROP, Jl Raya Tajur Km. 6 Bogor 16134, Indonesia
3
SEAFAST Center, IPB University, Darmaga Campus, Bogor 16680, Indonesia
Keywords: Aspergillus flavus, Aflatoxin, Toxigenic A. flavus, HPLC.
Abstract: Infection of toxigenic A. flavus in agricultural commodities may result in production of aflatoxin, a mycotoxin
which is genotoxic carcinogenic for humans and animals. The aims of this study were to screen toxigenic A.
flavus strains and to determine aflatoxin content of six agricultural commodities in Indonesia. A total of 50 A.
flavus strains were obtained from Phytopathology Laboratory, SEAMEO BIOTROP. The strains were isolated
from nutmeg, corn, cacao, white pepper, coffee bean, ground peanut and peanut-cropped soil. The toxigenicity
of A. flavus were determined bfy growth simulation on aflatoxin-inducing medium (10% coconut agar
medium) followed by observation of their fluorescence using 365 nm UV light. AFB and AFG toxin produced
were quantified using HPLC. The results showed that 18% (9 strains) A. flavus were toxigenic, which derived
from nutmeg (5 strains), ground peanut (2 strains), cacao (1 strain), and peanut-cropped soil (1 strain). Six
toxigenic strains produced AFB1 exceeding the Indonesian-regulatory maximum level (15 ug/kg). A. flavus
from peanut-cropped soil (BIO 3352) produced the highest AFB1 content (90.94 ug/kg), while the other from
nutmeg (BIO 3345 and BIO 33212), ground peanut (BIO 3313 and BIO 3338), and cacao (BIO 33404) had
AFB1 content of 89.53, 84.24, 70.26, 40.27, and 69.06 ug/kg respectively. The producing aflatoxin capability
of these strains can be potentially hazard if contaminated in agricultural commodities.
1 INTRODUCTION
Aflatoxins are secondary metabolites that produced
by Aspergillus section Flavi, particulary A. flavus and
A. paraciticus (Ellis et al, 1991). Natural occurrence
of aflatoxin in agricultural product lead to severe
health problems for human and livestock. Aflatoxins
confirmed as a Group-1 agent which is carcinogenic
to humans (IARC, 2012). Exposure to higher levels
of aflatoxin increases cancer incidence, including risk
of hepato-cellular carcinoma and neural tube defect
(Sun et al, 2011 and Woo et al, 2011). The Food and
Agricultural Organization (FAO) has been estimated
that approximately 25% of crops worldwide get
contaminated by mycotoxin producing fungi
including A. flavus, that contributing to global losses
of 1000 million metric tons foodstuffs each year
(Bhat et al, 2010). The contamination by
mycotoxigenic fungi can occur during harvest,
postharvest, storage and transportation and causes
significant economic losses yearly (Hedayati et al,
2007 and Nurtjahja et al, 2017).
Aflatoxins have a high occurrence in tropical and
subtropical regions due to optimal humidity and
temperature conditions for toxin production (Bhat et
al, 2010). Contamination of aflatoxin in agricultural
commodities was reported in many countries. Mandel
(2005) had reported that A. flavus was the dominant
fungi in contaminated nutmeg imported from India,
Sri Lanka, Indonesia and Brazil. Aflatoxins and
fumonisins were reportedly widespread in major
dietary and export targeted crops such as maize and
peanuts in Southern Africa (Hove et al, 2016;
Mwalwayo et al, 2016). According to Davari et al.,
(2015), out of 28 strains of A. flavus and A.paraciticus
isolated from 110 feed samples in northeastern Iran,
10 strains were toxigenic.
Indonesia’s agricultural commodities including
maize, peanut, pepper, nutmeg, and cacao have been
reported contaminated by aflatoxin (Nurtjahja et al,
2017; Dharmaputra, 2002; Dharmaputra et al, 2013).
About 54% of isolated fungi from stored nutmeg in
North Sulawesi, was identified as A. flavus with highest
Anidah, ., Rahayu, W. and Nurjanah, S.
Screening of Toxigenic Aspergillus flavus Strains and Aflatoxin Content from Agricultural Commodities in Indonesia.
DOI: 10.5220/0009981200002964
In Proceedings of the 16th ASEAN Food Conference (16th AFC 2019) - Outlook and Opportunities of Food Technology and Culinary for Tourism Industry, pages 221-226
ISBN: 978-989-758-467-1
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
221
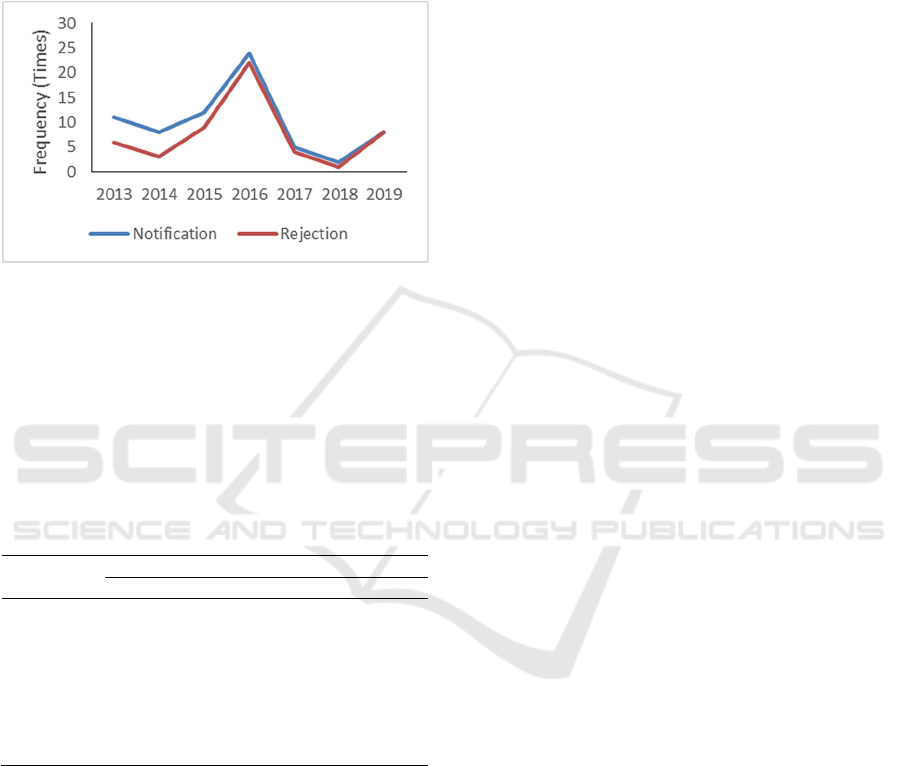
level of AFB1 and total aflatoxin content of 1.63 ppb and
1.83 ppb respectively (Dharmaputra et al, 2015).
According to Indonesian Food Security Agency
(Badan Ketahanan Pangan/BKP) from unpublish data
noted there was still rejection for Indonesia’s nutmeg
commodities until 2019 due to aflatoxin
contamination (Figure 1).
Figure 1: Notification and rejection of Indonesia’s nutmeg
export commodity (BKP, unpublish data).
The data from annual reports of Rapid Alert
System for Food and Feed (RASFF) in the last decade
showed mycotoxin alert notification in food which
aflatoxin were predominantly notified each year
(Table 1). Nuts and nut products were addressed as
notified contaminated food product every year.
Table 1: RSAFF notification on mycotoxin and aflatoxin in
food from 2010 – 2017.
Year
Notification
Mycotoxin Aflatoxin
2010 688 649 (94.3%)
2011 635 585 (92.1%)
2012 528 484 (91.7%)
2013 410 341 (83.2%)
2014 359 314 (87.5%)
2015 475 421 (88.6%)
2016 489 360 (73.6%)
2017 529 416 (78.6%)
There were many researches have been conducted
biological control strategy recently involving
toxigenic and atoxigenic A. flavus to reduce aflatoxin
contamination (Doner et al, 2003; Yin et al, 2009).
The information about characterization toxigenic as
well as atoxigenic were required as prelude for
choosing suitable strains for biological control. There
is minimum information about diversity of A. flavus
isolated from agricultural product in Indonesia. The
aims of this study were to screen toxigenic A. flavus
strains and to determine aflatoxin content from six
agricultural commodities in Indonesia. The
information about molecular characterization can be
used as further information for controlling aflatoxin
contamination using screened A. flavus.
2 MATERIAL AND METHODS
2.1 Aspergillus flavus Strain
The total of 50 strains of A. flavus were selected
randomly and were kindly provided by Fitophatology
Laboratory of SEAMEO BIOTROP, Indonesia
(Table 2). The strains were isolated from nutmeg,
corn, cacao, white pepper, coffee bean, ground peanut
and peanut-cropped soil from various regions in
Indonesia. BIO 747 strain was used as positive
control that can produced both AFB and AFG from
previous study (Nagur et al, 2014). Fungal cultures
were routinely subcultured on potato dextrose agar
(PDA: 39 g l-1, Difco Laboratories, Sparks, USA)
every two years.
2.2 Screening of Toxigenic A. flavus
Strains
For screening toxigenicity of A. flavus, all strains
were cultured on aflatoxin-inducing medium, 10%
(v/v) coconut agar medium (CAM, 100 mL fresh
shredded coconut endosperm, 900 mL distilled water,
15 g bacto agar, pH 7.0). A small amount of A. flavus
mycelium transferred into the centre of CAM and
incubated at 27
0
C for 5 days in the dark condition.
Observation of presence or absence of blue
fluorescence in the agar surrounding the A. flavus
colonies was determinated by exposing the petri dish
to long-wave (365 nm) UV light and expressed as
positive or negative toxigenicity. An uninoculated
plate was used as reference (Nurtjahja et al, 2017;
Davis et al, 1987). All the positive toxigenic strain
was further confirmed for aflatoxin quantification by
HPLC, along with positive control (BIO 747) and one
atoxigenic strain from screening as reference.
2.3 Aflatoxin Extraction and
Quantification by HPLC
Aflatoxin production simulated on 10% (v/v) coconut
broth (CB, 100 mL fresh shredded coconut
endosperm, 900 mL distilled water, pH 7.0) medium.
As much as 2 inoculum (ϕ 5mm) of each strains were
inoculated on 50 mL 10% (v/v) CB medium with
continuous shaking at 100 rpm (27
0
C, 10 days) in the
dark condition.
16th AFC 2019 - ASEAN Food Conference
222

Table 2: Expression of fluorescence and contents of aflatoxin from 50 A. flavus strains.
(na) not applicable; (+) fluorescence observed; (-) no fluorescence observed
(<) below the LoQ, for AFB1 = 1.42 ug/kg, AFB2 = 6.72 ug/kg, AFG1 = 5.09 ug/kg, and AFG2 = 0.66 ug/kg.
Commodities
(origin)
A. flavus
Strains
Fluorescence
in CAM
Aflatoxin (ug/kg)
AFB1 AFB2 AFG1 AFG2 AF-total
Nutmeg
(Manado – North Sulawesi)
BIO 3316 - na na na na na
BIO 3345 + 89.53 < < < 89.53
BIO 33184 - na na na na na
BIO 33212 + 84.24 < < < 84.24
BIO 33402 - na na na na na
BIO 33403 + 4.48 3.02 2.82 0.82 11.14
BIO 33211 + 5.03 < < < 5.03
BIO 3376 + 6.47 < 26.71 < 33.18
BIO 33185 - na na na na na
BIO 35102 - na na na na na
Coffee bean
(Jember – East Java)
BIO 3314 - na na na na na
Coffee bean
(Toraja – South Sulawesi)
BIO 3384 - na na na na na
BIO 3393 - na na na na na
BIO 3394 - na na na na na
BIO 3396 - na na na na na
Corn
(Bogor – West Java)
BIO 3382 - na na na na na
BIO 3311 - na na na na na
BIO 35111 - na na na na na
Cacao
(Makasar – South Sulawesi)
BIO 3312 - na na na na na
BIO 33404 + 69.06 < < < 69.06
BIO 33405 - na na na na na
White pepper
(Bogor, West Java)
BIO 3383 - na na na na na
BIO 3316 - na na na na na
BIO 25119 - na na na na na
Ground peanut
(Bogor – West Java)
BIO 3313 +
70.26
6.59 < 1.01 77.86
BIO 3381 - < < < < <
BIO 3346 - na na na na na
BIO 3348 - na na na na na
Ground peanut
(Wonogiri – Central Java)
BIO 3342 - na na na na na
BIO 3324 - na na na na na
BIO 3334 - na na na na na
BIO 3338 + 40.27 < 97.28 < 137.55
BIO 3340 - na na na na na
BIO 3341 - na na na na na
BIO 3322 - na na na na na
BIO 3325 - na na na na na
BIO 3324 - na na na na na
Peanut-cropped soil
(Wonogiri – Central Java)
BIO 3352 + 90.94 < < < 90.94
BIO 3362 - na na na na na
BIO 3364 - na na na na na
BIO 3367 - na na na na na
BIO 3374 - na na na na na
BIO 3378 - na na na na na
BIO 3386 - na na na na na
BIO 3387 - na na na na na
BIO 3390 - na na na na na
BIO 3357 - na na na na na
BIO 3391 - na na na na na
BIO 3392 - na na na na na
BIO 3357 - na na na na na
Toxigenic strain BIO 747
+
74.01 < 54.05 < 128.06
Screening of Toxigenic Aspergillus flavus Strains and Aflatoxin Content from Agricultural Commodities in Indonesia
223

Total aflatoxin was extracted from ten-days-old 10%
(v/v) CB medium cultures of toxigenic strains, using
AOAC method 991.3125,26. A 25 ml of filtered
extract was pipetted and extracted with 5 g NaCl and
125 ml of methanol:water (70:30) ratio into blender
jar, and blended for 2 minutes at maximum speed.
The filtered extract (15 ml) was diluted with 30 ml of
purified water into a clean vessel. The diluted extract
was filtered through glass microfiber filter. A 15 ml
filtered diluted extract passed completely through
AflaTest affinity column (VICAM, USA) at a rate of
about 1-2 drops/second and washed with 2 x 10 ml of
purified water at a rate of 2 drops/second. Total
aflatoxin was eluted from column with addition of 1
ml HPLC grade methanol (Merck, Germany) at rate
of 1 drop/second. Eluted sample was collected in a
glass cuvette and added with 1 ml deionized water.
Afterward, 20 ul of eluate were injected onto HPLC.
Chromatographic analyses were performed with
an Agilent 1260 Infinity Isocratic LC (Agilent
Technologies, USA), equipped with Photochemical
Reactor Derivatization (AURA Industries).
Excitation and emission wavelengths were 365 and
465 nm respectively. A Bonclone 10u C18 Column
(Phenomex, 3.9 x 150 mm) was used. The mobile
phase was methanol: water (60:40) and the flow rate
was 1.3 ml/min. Injection volume was 20 ul.
Quantification of aflatoxin was perfomed by
comparing the peak areas with the calibration curves
of each aflatoxin.
3 RESULT AND DISCUSSION
The screening on aflatoxin-induced medium (CAM)
was initially used to identify the aflatoxin production
from 50 A. flavus strains by fluorescence observation,
revealed that nine strains (18%) were toxigenic
(Table 2).
Figure 2: Fluorescence of A. flavus strains on 10% CAM.
(Left – right); uninoculated CAM, atoxigenic strain (BIO
3381), toxigenic strain (BIO 3313).
Toxigenic strains were originated from nutmeg
(56%), cacao (22%), ground peanut (11%), and
peanut-cropped soil (11%). Atoxigenic strains was
obtained from coffee bean, corn, and pepper. Blue
fluorescence was observed from outside of the colony
in CAM from eight strains, meanwhile fluorescence
was not seen either from uninoculated media or
atoxigenic strain (Figure 2). The naturally presence of
toxigenic and atoxigenic A. flavus have been reported
in many studies. Wei et al, (2014) found by UPLC
detection, 76% of the 323 A. flavus strains isolated
from peanut field in four provinces in China, were
aflatoxins producer with limit of detection method
was 1 µg/kg.
All the toxigenic strains, also positive and
negative control strains were further confirmed by
measuring AFB dan AFG content by HPLC from
growth simulation in CB medium. BIO 3381 was
chosen as negative control as no fluorescent observed
in CAM. During the incubation process, mycelia
grew on the surface of the media, while the toxin
produced was dissolved in the media. The aflatoxins
content determined as AFB1, AFB2, AFG1, AFG2
and total aflatoxin. The result showed all toxigenic
strains produced AFB1 (Table 3). Six toxigenic
strains produced AFB1 exceeding the Indonesian-
regulatory maximum level (15 ug/kg). A. flavus from
peanut-cropped soil (BIO 3352) produced the highest
AFB1 content (90.94 ug/kg), while the other strains
from nutmeg (BIO 3345 and BIO 33212), ground
peanut (BIO 3313 and BIO 3338), and cacao (BIO
33404) had AFB1 content of 89.53, 84.24, 70.26,
40.27, and 69.06 ug/kg respectively.
Table 3: Summary of aflatoxin content of 9 toxigenic A.
flavus strains.
Commodities
A. flavus
Strains
Aflatoxin (ug/kg)
AFB1 AFB2 AFG1 AFG2
Nutmeg BIO33212 84.24 < < <
BIO33403 4.48 3.02 2.82 0.82
BIO33211 5.03 < < <
BIO3376 6.47 < 26.71 <
BIO3345 89.53 < < <
Cacao BIO33404 69.06 < < <
Ground
peanut
BIO3313 70.26 6.59 < 1.01
BIO3338 40.27 < 97.28 <
Peanut-
cropped soil
BIO3352 90.94 < < <
(<) below the LoQ, for AFB1: 1.42 ug/kg, AFB2: 6.72
ug/kg, AFG1: 5.09 ug/kg, and AFG2: 0.66 ug/kg.
There was only one strain (BIO 33403) that
produced all aflatoxins types. Meanwhile one strains
(BIO 3313) produce all aflatoxins types except
AFG1, and two strains (BIO 3338 and BIO 3376)
produce AFB1 and AFG1. Five strains observed
16th AFC 2019 - ASEAN Food Conference
224

which only produced AFB1 were BIO 3345, BIO
3352, BIO 33211, BIO 33212, and BIO 33404. A.
flavus had known as AFB producer and A. paraciticus
as AFG producer which were determined by the color
of fluorescence of the colony on 10% CAM
(Nurtjahja et al, 2017). This study found that 44.4%
strains of toxigenic A. flavus can produce either AFB
or AFG.
In this study, strain isolated from peanut-cropped
soil was the higher production of AFB1. According
to Pitt (1989) in Dharmaputra et al., (2001), A. flavus
and A. paraciticus are present in high numbers in
cultivated soils. They are able to grow as commensals
in developing peanut plants, and start to invade
developing peanuts (Pitt et al., 1991). The study of
soil isolates and the correlation with toxigenicity
potential was reported by Dharmaputra et al., (2002).
She reported that 44% of toxigenic A. flavus were
identified from 48 soil sample during wet season, and
51% during dry season, in Pati regency (Central
Java). Most of the toxigenic A. flavus produced AFB1
and AFB2 and some of them produced AFB1, AFB2,
AFG1, and AFG2. Toxigenic A. flavus also found as
much as 27.5% from 66 strains isolated from corn
field soil in Iran, and only produce AFB1 or AFB1
and AFB2 (Razzaghi-Abyaneh et al., 2006).
4 CONCLUSIONS
Nine strains of toxigenic A. flavus were obtain from
screening of 50 strains from 6 agriculture
commodities and 1 peanut-cropped soil in Indonesia
which can produced aflatoxin. It was assumed that
soil from plantation could be a media for A. flavus
infection to the plant. The result of this study gave
information that toxigenic A. flavus strains have
ability to produce aflatoxin and could be used as
positive control in biological control. Further studies
are needed to characterize the diversity in DNA level
among the toxigenic strains. The information about
molecular characterization could help to develop
more effective biological control strategy.
ACKNOWLEDGEMENTS
The authors would like to acknowledge SEAMEO
BIOTROP for providing financial support through
Daftar Isian Pelaksanaan Anggaran (DIPA) 2019.
Thanks, are also to Prof. Dr Okky Setyawati
Dharmaputra and Ms. Ina Retnowati, S. Si for their A.
flavus collection isolates.
REFERENCES
AOAC (Association of Official Analytical Chemists).,
2000. Section 49.2.18 (AOAC Method 991.31) for
corn, raw peanut, and peanut butter. In Official Method
of Analysis, Seventeenth Edition. Gaithersburg, MD,
USA, AOAC International.
Bhat, R., Rai, R. V., & Karim, A. A., 2010. Mycotoxins in
Food and Feed: Present Status and Future Concerns.
Comprehensive Reviews in Food Science and Food
Safety. (9): 57-81.
Davari E., Mohsenzadeh M., Mohammadi Gh., Rezaeian-
Doloei R., 2014. Characterization of aflatoxigenic
Aspergillus flavus and A. paraciticus strain isolates
from animal feedstuffs in northeastern Iran. IJVR. 16
(2): 150-155.
Davis N.D., Iyer S.K., Diener U.L., 1987. Improved method
of screening for aflatoxin with a coconut agar medium.
Appl. Environ. Microbiol. 1987; 53(7):1593-1595.
Dharmaputra O.S., 2002. Review on aflatoxin in
Indonesian food and feedstuffs and their products.
Biotropia. 19:26 – 46.
Dharmaputra O.S., Ambarwati S., Retnowati I., Nurfadila
N., 2015. Fungal infection and aflatoxin contamination
in stored nutmeg (Myristica fragnans) kernels at
various stages of delivery chain in north Sulawesi
province. Biotropia. 22(2): 129-139.
Dharmaputra O.S., Ambarwati S., Retnowati I.,
Windyarani A., 2013. Aspergillus flavus population and
aflatoxin B1 content in processed peanut product in
municipality of Bogor, West Java, Indonesia. Biotropia.
20(2): 81-88.
Dharmaputra O.S., Putri A.S.R., Retnowati I., Ambarwati
S., 2001. Soil mycobiota of peanut fields in Wonogiri
regency, Central Java: Their effect on the growth and
aflatoxin production of Aspergillus flavus in vitro.
Biotropia. (17): 30-59.
Dharmaputra O.S., Retnowati I., Ambarwati S. Toxigenic
Aspergillus flavus isolates in the soil of peanut farms in
Pati regency, Central Java. 2002b. Bogor: SEAMEO
BIOTROP.
Doner J.W., Cole R.J., Cinnick W.J., Daiglle D.J., McGuire
M.R., Shashad B.S., 2003. Evaluation of biological
control formulation to reduce aflatoxin contamination
in peanuts. Biol Control. (38):329-339.
Ellis W.O., Smith J.P., Simpson B.K., 1991. Aflatoxin in
food: occurrence, biosynthesis, effect on organism,
detection and method of control. Critical Review in
Food Science and Nutrition. (30): 403-439.
Hedayati M.T., Pascualotto A.C., Warn P.A., Bowyer P.,
Denning D.W., 2007. Aspergillus flavus: human
pathogen, allergen and mycotoxin producer. Review.
Microbiologi. 153: 1677 – 1692.
Hove M.C., Van Poucke E., Njumbe-Edjage L.K., Nyanga,
S. De Saeger., 2016. Review on the natural co-
occurance of AFB1 and FB1 in maize and the combined
toxicity of AFB1 and FB1. Food Control. (59): 675-82.
IARC (International Agency for Research on Cancer),
2012. Monograph on the evaluation of carcinogenic
Screening of Toxigenic Aspergillus flavus Strains and Aflatoxin Content from Agricultural Commodities in Indonesia
225

risk to human. Chemical agent and related occupations.
IARC Press. Lyon, France, Volume 100 F.
Mandel Q.A., 2005. Fungal contamination of some
imported spices. Mycopathologia. 59: 291-8.
Mwalwayo D. S., and B. Thole., 2016. Prevalence of
aflatoxin and fumonisins (B1 & B2) in maize consumed
in rural Malawi. 2016. Toxicology Reports. (3):173–9.
Nagur K. S., Sukarno N., Listriyowati S. 2014.
Identification of Aspergillus flavus and detection of its
aflatoxin genes isolated from peanut. Biotropia. 21(1):
64-75.
Nurtjahja K., Dharmaputra O.S., Rahayu W.P., 2017.
Gamma irradiation of Aspergillus flavus strains
associated with Indonesian nutmeg (Myristica
fragrans). Food Sci Biotechnol. 26(6):1755-1761.
Pitt J.I., Dyer S.K. and McCammon S., 1991. Systemic
invasion of developing peanut plant by Aspergillus
flavus. Letters in Applied Microbiology. (13):16-20.
Pitt, J.I., 1989. Field studies on Aspergillus and aflatoxins
in Autralian groundnuts. In McDonald D. and Mehan
V.K. Aflatoxin contamination of groundnut.
Proceeding of International Workshop, 6-9 October
1989, ICRISAT Center, India. p. 223-235.
RSAFF., 2011. The Rapid Alert System for Food and Feed
2010 Annual report. European Comission.
RASFF., 2012. The Rapid Alert System for Food and Feed
2011 Annual report. European Commission.
RASFF., 2013. The Rapid Alert System for Food and Feed
2012 Annual report. European Commission.
RASFF., 2014. The Rapid Alert System for Food and Feed
2013 Annual report. European Commission.
RASFF., 2015. The Rapid Alert System for Food and Feed
2014 Annual report. European Commission.
RASFF., 2016. The Rapid Alert System for Food and Feed
2015 Annual report. European Commission.
RASFF., 2017. The Rapid Alert System for Food and Feed
2016 Annual report. European Commission.
RASFF., 2018. The Rapid Alert System for Food and Feed
2017 Annual report. European Commission.
Razzaghi-Abyaneh M., Shams-Ghahfarokhi M., Allameh
A., Kazeroon-Shiri A., Ranjbar-Bahadori S.,
Mirzahoseini H., Rezaee M., 2006. A survey on
distribution of Aspergillus section flavi in corn field
soils in Iran: population patterns based on aflatoxins,
cyclopiazonic acid and sclerotia production.
Mycopathologia. (161): 183-192.
Sun, G., Wang, S., Hu, X., Su, J., Zhang, Y., Xie, Y., Zhang,
H., Tang, L., & Wang, J. S., 2011. Co-contamination of
aflatoxin B1 and fumonisin B1 in food and human
dietary exposure in three areas of China. Food Addit
Contam. Part A Chem Anal Control Expo Risk Assess.
(28): 461-470.
Wei D., Zhou L., Selvaraj J.N., Zhang C., Xing F., Zhao Y.,
Wang Y., Liu Y., 2014. Molecular Characterization of
Atoxigenic Aspergillus flavus Isolates Collected in
China. J. Microbiology. 52:559-565.
Woo, L. L., Egner, P. A., Belanger, C. R.,
Wattanawaraporn, R., Trudel, L. J., Croy, R. G.,
Groopman, J. D., Essigmann, J. M., & Wogan, G. N.,
2011. Aflatoxin B1-DNA adduct formation and
mutagenicity in livers of neonatal male and female
B6C3F1 mice. Toxicological Sciences. 122(1): 38 – 44.
Yin Y., Lou T., Yan L., Michailides T.J., M a Z., 2009.
Molecular characterization of toxigenic and atoxigenic
Aspergillus flavus isolates, collected from peanut fields
in China. Journal of Applied Microbiology. (107):
1857-1865.
16th AFC 2019 - ASEAN Food Conference
226
