
Purslane (Portulaca Oleracea L.) Leaves Extract Addition in Jelly
Candy Making
Ratna Handayani, Melina Christine and Bryan Anders
Food Technology Department, Universitas Pelita Harapan, Jl. M.H Thamrin Boulevard, Tangerang, Indonesia
Keywords: Candy, Jelly, Purslane, Linolenic Acid, Maceration.
Abstract: Purslane or krokot (Portulaca oleracea L.) can grow in warm climates (tropics and subtropics). Purslane
plant is known to contain 41.4-66.4% of the omega-3 fatty acids, linolenic fatty acids (C18:3, n-3). Purslane
extract contains unsaturated fatty acids, and more than 10 % consisted of alpha linolenic acid. This study is
done to utilize purslane extract in candy jelly production that can be accepted by panelists. Maceration is
used to extract purslane leave with three types of solvents (ethanol, ethyl acetate and hexane). The best
extract was obtained by calculating the total yield and GC-MS analysis of purslane leaf extract components.
The addition of purslane leaf extract to jelly candy uses concentrations of 0.5%, 1.0%, 1.5% and 2.0%
(w/w). The observed parameters include physicochemical analysis (Total Dissolved Solids and pH), color,
texture and sensory analysis. Selected jelly candies have a concentration of 0,5% purlsane leaf extract.
Selected jelly candy has characteristics including ° Hue of 91.54 °, Total Dissolved Solids of 34.68 ° Brix,
moisture content of 46.49%, fat content of 1.59%, protein content of 0.43%, ash content of 0.87 % and
carbohydrate levels of 50.72%. Then based on the results of GC-MS analysis the selected jelly candy
contains linolenic acid.
1 INTRODUCTION
Purslane (Portulaca olearacea L.), in Indonesia is
known by the name of krokot. Krokot is a type of
ornamental plant that can grow on warm climate. In
some countries (e.g. China), krokot is commonly
used in traditional medicine to treat diabetes and
hypotension; and it is also known for its antifungal,
antibiotic, antiinflammation, antiestrogenic, and
anticancer properties (Agil, et al., 2015; Uddin, et
al., 2014; Sultana dan Rahman, 2013; dan Zhou, et
al., 2015; Hanan et al., 2014). The leaves contain
41.4-66.4% (1.0-1.6 mg/g) omega-3 fatty acids,
which are higher compared to its root and stem.
Omega-3 fatty acid has many beneficial effects
to human body, namely its anticoagulative and anti-
inflammatory properties. It is also known to be good
for the brain. Moreover, omega-3 fatty acids are able
to prevent chronic diseases, such as cardiovascular
related diseases, hypertension, inheritance of
diabetes on fetus during pregnancy, inflammation,
hyperlipidemia, and cancer (Diana, 2012; Zhou, et
al., 2015; Yessoufou, et al., 2015; Calviello and
Serini, 2010).
Jelly
candy is a type of confectionary with soft,
chewy, and elastic texture. Gelatin used in the
making of jelly candies comes from connective
tissues of pig, cow, or skin from poultries
(Subaryono and Utomo, 2006; Riaz dan Chaudry,
2004). Due to its non-halal origin, other
hydrocolloids such as carrageenan is used as a
substitute for gelatin (Atmaka, Nurhartadi and
Karim, 2013).
This research is done to determine the addition of
krokot leaf extract in the production of jelly candies,
based on its sensory analysis and physic analysis.
2 MATERIALS AND METHODS
The materials used are fresh krokot leaves obtained
from BALITRO, Bogor, food grade solvents
(ethanol, ethyl acetate, and hexane), granulated
sugar, citric acid, kappa-carageenan, konjac, high
fructose syrup (HFS), and orange flavoring.
Materials used for analysis consist of distilled water,
pro-analysis grade hexane, K
2
SO
4
, selenium, H
2
SO
4
97%, H
2
O
2
, and saturated boric acid. The equipment
used are “Ohaus U-1800 AR 2140” analytical
Handayani, R., Christine, M. and Anders, B.
Purslane (Portulaca Oleracea L.) Leaves Extract Addition in Jelly Candy Making.
DOI: 10.5220/0009992400002964
In Proceedings of the 16th ASEAN Food Conference (16th AFC 2019) - Outlook and Opportunities of Food Technology and Culinary for Tourism Industry, pages 83-90
ISBN: 978-989-758-467-1
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
83

balance, heater, cabinet dryer, oven, Tyler sieve (35
mesh), “Buchi” rotary evaporator, “Konica Minolta
CR-400” chromameter, furnace, “Aglient 7890B”
Gas Chromatograph, “Aglient 5977B” Mass
Selective Detector, and desiccator.
2.1 Extraction of Krokot Leaf
The extraction of krokot leaf is done using
maceration method. The leaves are dried using
cabinet dryer at 50°C for 24 hours (Abdolshahi, et al.
(2015);Kaveh, et al. (2017), with modification). The
dried leaves are reduced in size and sieved using 35-
mesh Tyler sieve to obtain krokot leaf powder. The
powder is macerated using three types of solvent
(ethanol, ethyl-acetate, and hexane) with the ratio of
1:4 (w:v) powder to solvent for 72 hours. The liquid
is then filtered using Whattman No.1 filter paper and
the solvent is evaporated by using rotary evaporator
at 45°C with adjusted vacuum pressure based on each
solvent to obtain krokot leaf extract.
2.2 Production of Jelly Candy
The production of jelly candy is done by combining
the hydrocolloid (carrageenan and konjac) with
water at 80-90°C in a separate container for each
hydrocolloid. Then, in another container, mix high
fructose syrup with granulated sugar. When the
sugar has dissolved, mix the sugar and syrup mixture
with carrageenan and konjac solution, followed by
heating. When the mixture temperature reaches
80°C, add the citric acid and flavoring.
After cooling down to 60°C, krokot leaf extract
is added to the mixture (Sugiharto, et al., 2015). The
jelly candy mixture is then poured to the mold, that
has been applied with molding starch. The jelly
candy is left to cool down in room temperature (23-
30°C) for an hour before transferring to the
refrigerator (4-5°C) and left for 24 hours. During
refrigeration, the gel sets. Compared to konjac, the
gel from carrageenan sets faster. Carrageenan gel
sets at around 40-70°C. However, its setting
temperature is highly affected by the sugar content
of the jelly candy, thus it is hard to determine the
specific set temperature for carrageenan (Williams
dan Phillips, 2004; Williams and Phillips
1
, 2009).
2.3 Characterization of Krokot Leaf
Powder
Characterization of krokot leaf powder was done to
determine the chemical composition through
proximate analysis (AOAC,2015)
2.4 Characterization of Krokot Leaf
Extract
The extracts from three different solvent was
calculated for its yield using the formula:
Yield (%) =
100%
Furthermore, the identification of omega-3 fatty acid
is determined using GC-MS.
2.5 Analysis of Krokot Leaf Extract
Added Jelly Candy
The produced jelly candy will be analysed for its pH
(AOAC, 2005), Total Soluble Solid (Hasyim 2015),
Color (Nielsen, 2010), Texture (Azizah 2012),
Sensory Evaluation (Lawless and Heymann, 2010;
Kemp, et al. 2009), and proximate analysis (AOAC
2015).
3 RESULTS AND DISCUSSION
3.1 Characteristics of Krokot Leaf
Powder
The chemical composition of krokot leaf powder is
shown in Table 1. Based on the analysis done, the
moisture content of the krokot leaf powder is
8.44±0.13%, which according to Puspitasari and
Proyogo (2017), the moisture content of dry plant
sample must not exceed 10%. Thus, krokot leaf
powder obtained can be used for the next step,
maceration.
Table 1: Krokot leaf chemical composition.
Chemical composition Result (%)
Moisture content 8.44 ± 0.13
Fat 3.96 ± 0.07
Protein 31.75 ± 0.06
Ash 18.24 ± 0.03
Carbohydrate 37.60 ± 0.03
3.2 Characterization of Krokot Leaf
Extract
Based on the extract yield on Table 2., food grade
ethanol gives the highest yield among the solvents
used, with the value of 8.30±0.02%. Similar result is
obtained from a research by Adiyasa, Wrasiati and
Wartini (2015), where ethanol gives the highest
extract yield.
16th AFC 2019 - ASEAN Food Conference
84
Based on the component identification result
with GC-MS, all solvent used shows similar result
qualitatively, where every extract contains omega-3
fatty acid. Table 3. shows the identification result
Table 2: Krokot leaf extract yield.
Solvent Type Yield (%)
Ethyl-acetate 4.78±0.14
Ethanol 8.30±0.02
Hexane 2.99±0.01
In a research done by Schmid, et al., (2016),
ethanol gives higher PUFA (Polyunsaturated Fatty
Acid) yield in the extraction of seaweed.
Furthermore, ethanol solvent is able to extract higher
amount of total fatty acid (TFA). Hence, it is
possible that the krokot leaf extract obtained by
using ethanol as solvent contain high level of PUFA
and TFA. Thus, ethanol is chosen as the best solvent
for the extraction of krokot leaf.
Table 3: Total component identification of Krokot leaf
extract with GC-MS.
Solvent Type Retention Time Qual
Ethyl-acetate 10.314 minutes 98%
Ethanol 10.968 minutes 99%
Hexane 10.540 minutes 94%
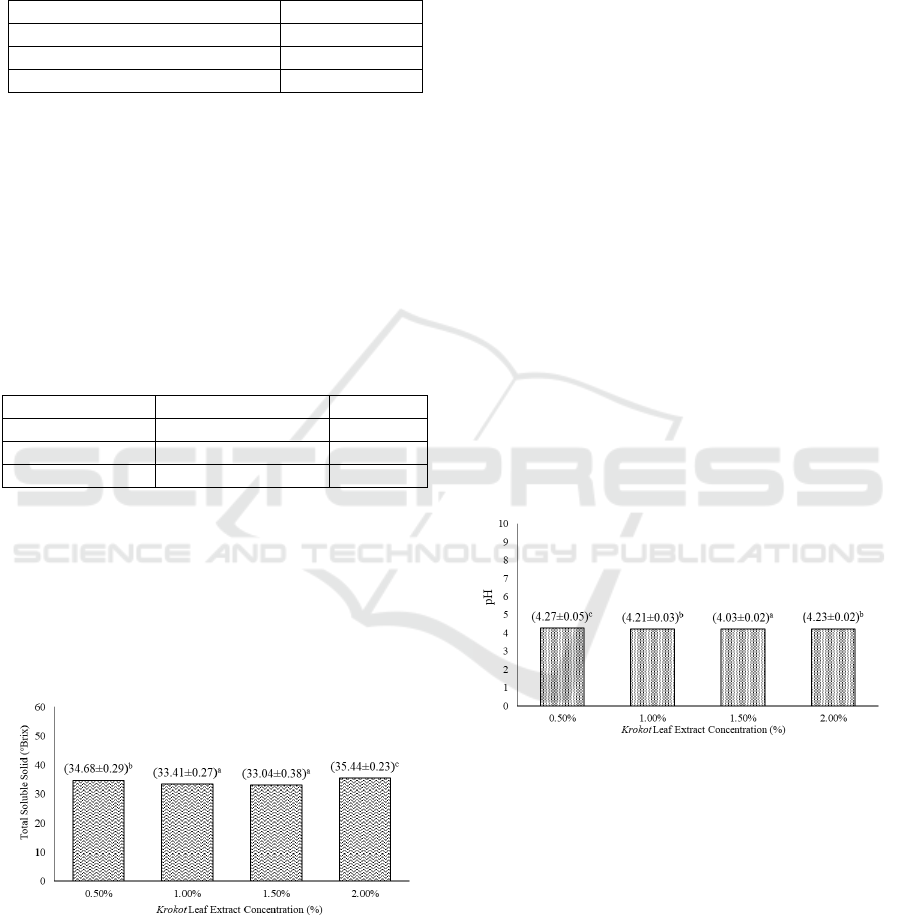
3.3 Effect of Krokot Leaf Extract
Concentration on Total Soluble
Solid of Produced Jelly Candy
Based on Figure 1, there is a significant difference in
total soluble solids between jelly candy with
different level of extract concentration.
Figure 1: Effect of extract concentration on the value of
jelly candy total soluble solid. (Note: Different letter
notations indicates a significant difference at p≤0.05).
It can be seen that there is an increasing trend of
total soluble solid when higher level of extract is
added. Similar trend is seen on a research by
Charoen, et al. (2015), where the total soluble solid
of the jelly candy produced increases as the level of
guava leaf extract is increased. Another possibility is
that increase in total soluble solid is caused by the
bonding of free water and other particle through
stabilizer, thus increasing the total soluble solid and
reducing formation of precipitate (Farikha, et al.,
2013).
3.4 Effect of Krokot Leaf Extract
Concentration on pH of Produced
Jelly Candy
Based on Figure 2., there is a significant difference
in pH value between jelly candies with different
levels of krokot leaf extract. In Figure 2, an inverse
relationship can be seen on the pH of jelly candy and
the addition of krokot leaf extract. This is due to the
addition of citric acid in the making of jelly candy.
The decrease in pH can also be explained due to the
addition of carrageenan (Septiani, et al. (2013). The
presence of anhydrogalactose group on carrageenan
decreases the dispersion of carrageenan, thus
reducing the (H
+
) ion that is bound in the jelly
candy, effectively reducing the pH of the product.
The jelly candies from every treatment is
categorized as acidic to less than 7 pH value.
According to Sperbe and Doyle (2009), pH value in
candies have range between 2.0 until 8.0.
Figure 2: Effect of extract concentration on the value of
jelly candy pH. (Note: Different letter notations indicates
a significant difference at p≤0.05).
3.5 Effect of Krokot Leaf Extract
Concentration on Color of
Produced Jelly Candy
The °Hue value of the candy can be seen on Figure
3, where there is no significant difference on °Hue
value between jelly candy with different
concentration of krokot leaf extract. The °Hue value
of the product ranges between 86.91 to 92.52°,
where according to Munsell color system, the jelly
candy color is categorized as yellow. Increasing the
Purslane (Portulaca Oleracea L.) Leaves Extract Addition in Jelly Candy Making
85
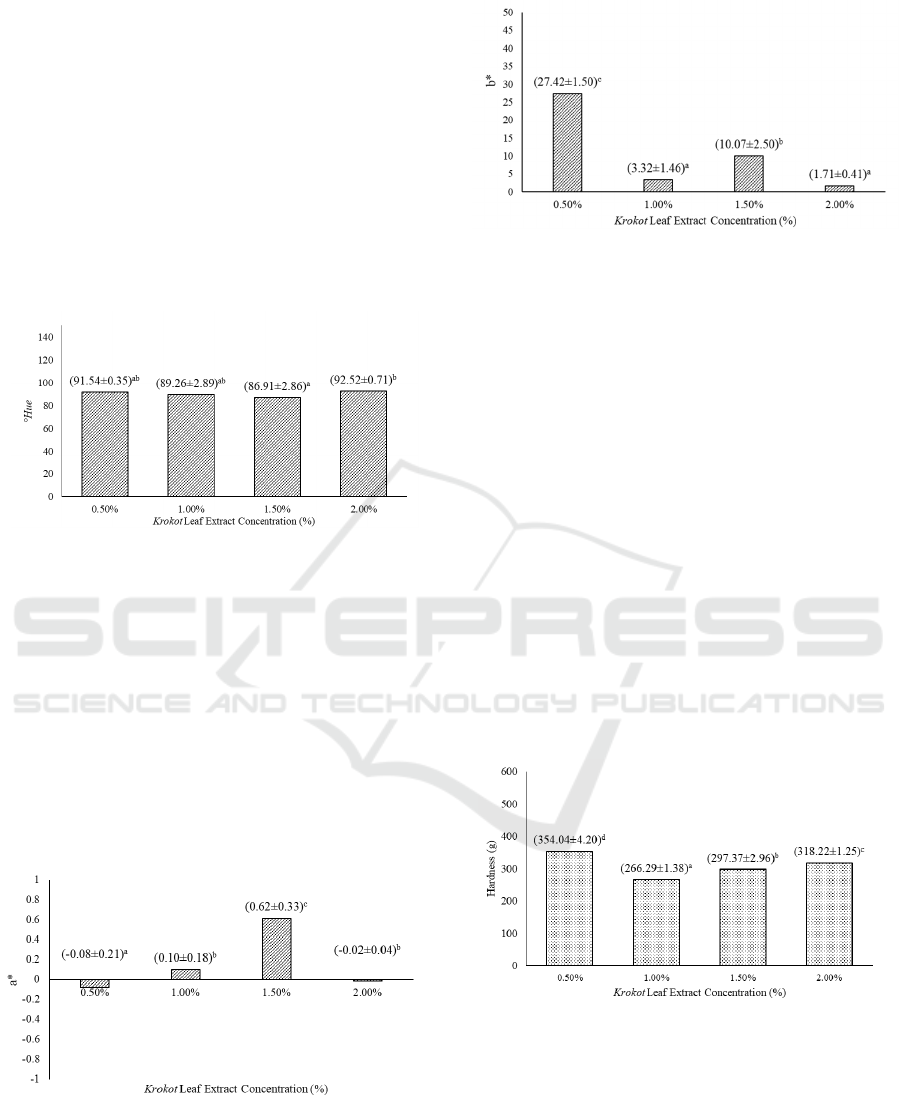
concentration of krokot leaf extract will increase the
°Hue value of the jelly candy. However, the result
obtained is different from visual observation, where
the candy shows dark green color. This is due to the
presence of antioxidant in the extract used.
According to a research by Liu, et al. (2000), krokot
contains high level of antioxidant in form of beta-
carotene (22-30 mg/g). MacDougall (2002) said that
beta-carotene gives yellow orange to food product.
According to USDA (2015), a cup of raw carrot
contains 10605 µg or 10.6 mg beta-carotene. Due to
high level of beta-carotene in the krokot leaf extract,
it affects color perceived as yellow.
Figure 3: Effect of extract concentration on the value of
jelly candy
o
Hue. (Note: Different letter notations
indicates a significant difference at p≤0.05).
In color analysis, °Hue value is directly affected
on the values a* and b*. Based on Figure 4 and
Figure 5, the range of a* and b* value for the candy
is between -0.08 to 0.61 and 1.71 to 27.42
respectively. From the result, it can be perceived that
every jelly candy has different color. Lower a* value
indicates green color. While higher b* value
indicates yellow and lower b* value will lean
towards blue color (Nielsen, 2010). Because of this,
°Hue value obtained leans toward yellow color.
Figure 4: Effect of extract concentration on the value of
jelly candy a* value. (Note: Different letter notations
indicates a significant difference at p≤0.05).
Figure 5: Effect of extract concentration on the value of
jelly candy b*. (Note: Different letter notations indicates a
significant difference at p≤0.05).
3.6 Effect of Krokot Leaf Extract
Concentration on Texture of
Produced Jelly Candy
Texture is an important parameter when determining
the physical characteristic of jelly candy. The type
and concentration of gelling agent used will
determine the texture of jelly candy produced
(Imeson, 2010). The observed parameters used in
texture analysis of jelly candy are hardness,
springiness, cohesiveness, chewiness, and
gumminess.
Based on Figure 6, krokot leaf extract has a
significant effect towards the hardness of jelly candy
produced. Hardness value tend to decrease as the
lever of extract is increased. Lower hardness value
indicates softer texture, and vice versa (Octaviana,
Purwijantiningsih and Pranata, 2013).
Figure 6: Effect of extract concentration on the value of
jelly candy hardness. (Note: Different letter notations
indicates a significant difference at p≤0.05).
Decrease in hardness can also due to jelly candy,
which considered hygroscopic, absorbs water when
exposed to environment with high humidity and
temperature, making the product softer when
analyzed (Koswara, 2009; Rahmi, et al., 2012).
Based on Figure 7., no significant difference is
shown between different level of extract
16th AFC 2019 - ASEAN Food Conference
86
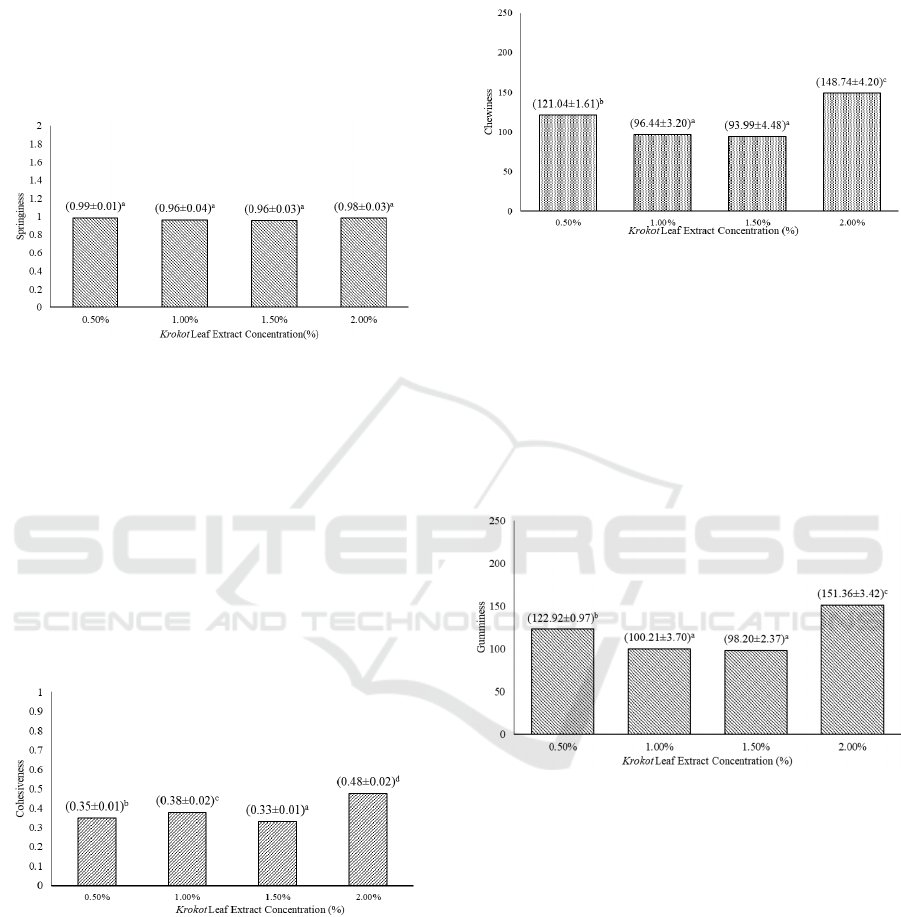
concentration towards the springiness of jelly candy.
Springiness of jelly candy is usually not affected by
the addition of different levels of extract
concentration. Similar result is shown in a research
by Atmaka, Nurhartadi and Karim (2013), where
different Curcuma zanthorrhiza extract
concentration shows no significant effect to the
elasticity of jelly candy produced.
Figure 7: Effect of extract concentration on the value of
jelly candy springiness. (Note: Different letter notations
indicates a significant difference at p≤0.05). at p≤0.05).
Based on Figure 8., there is a significant
difference towards the cohesiveness of jelly candy
between different levels of extract. Cohesiveness
value of jelly candy tends to increase as the amount
of extract added is increased. The increase and
decrease in cohesiveness value is affected by things
like moisture content, sugar, and other minor
components, which may affect the molecular bond
of the gel structure (Rahmi, et al., 2012; Delgado
and Bañón, 2014).
Figure 8: Effect of extract concentration on the value of
jelly candy cohesiveness. (Note: Different letter notations
indicates a significant difference at p≤0.05).
As seen on Figure 9., there is a significant
difference of jelly candy chewiness between
different levels of extract concentration. A research
by Purwaningtyas, Suhartatik and Mustofa (2017),
however, shows no effect on chewiness from the
addition of suji and sirih leaf exract. The increase in
chewiness of the jelly candy produced may be
attributed by the moisture content, total soluble
solids, and gelling agent working synergistically
(Delgado and Bañón, 2014).
Figure 9: Effect of extract concentration on the value of
jelly candy chewiness. (Note: Different letter notations
indicates a significant difference at p≤0.05).at p≤0.05).
Despite increasing value, the gumminess of jelly
candy produced shows no significant difference
between different levels of extract added.
Gumminess shows gel strength, where it is affected
by moisture content, total soluble solids, and
presence of other minor components. (Delgado and
Bañón, 2014; Rahmi, et al., 2012).
Figure 10: Effect of extract concentration on the value of
jelly candy guminess. (Note: Different letter notations
indicates a significant difference at p≤0.05). at p≤0.05).
3.7 Effect of Krokot Leaf Extract
Concentration on Sensory
Evaluation of Produced Jelly
Candy
As shown on Table 4., higher extract concentration
shows an increase score in color albeit not
significant. Higher level of extract will show greener
color jelly candy.
Purslane (Portulaca Oleracea L.) Leaves Extract Addition in Jelly Candy Making
87
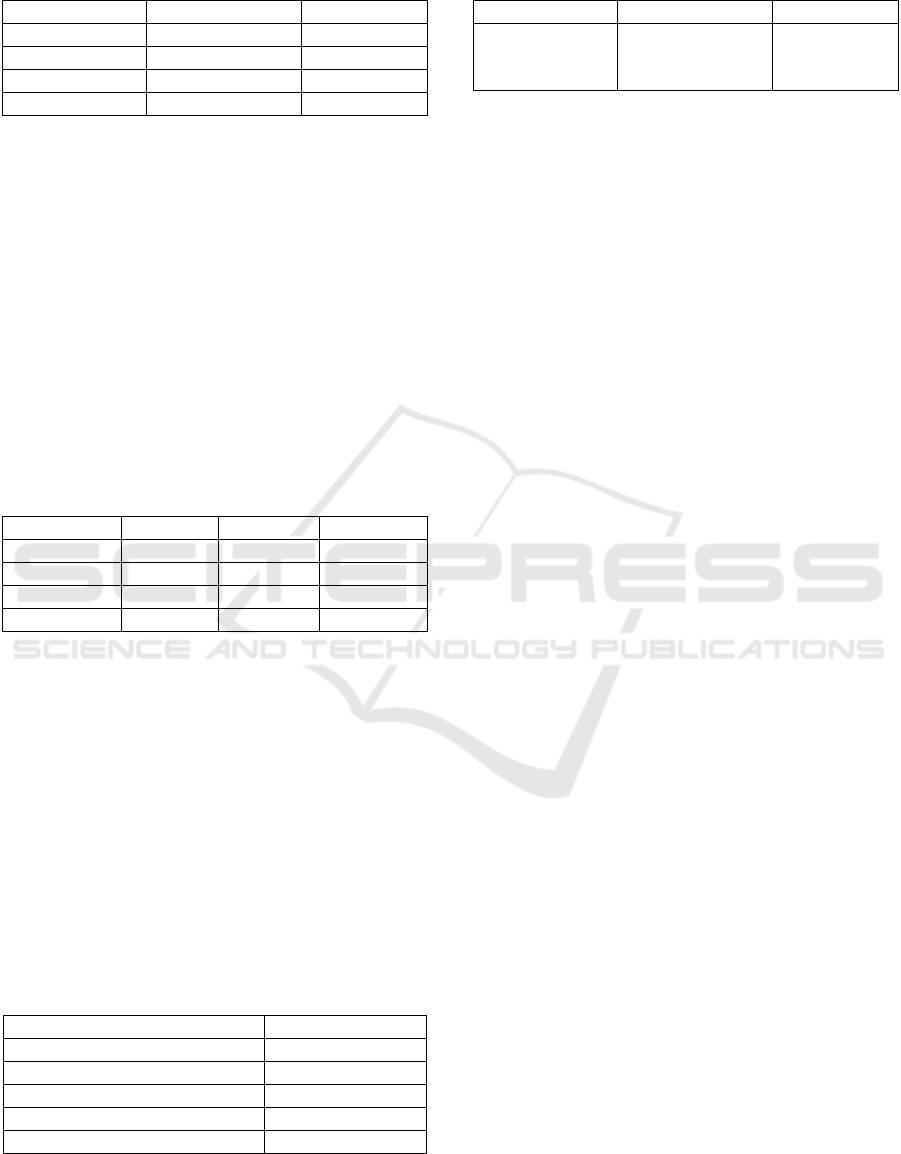
Table 4: Effect of Krokot Leaf Extract to scoring of jelly
candy.
Concentration Color Flavor
0.5% 4.04±1.9
a
4.40 ±1.03
c
1% 5.11±1.22
b
3.53±1.10
b
1.5% 5.00±1.15
b
3.33±1.40
b
2% 5.36±1.12
b
2.89±1.48
a
Addition of higher extract concentration exhibit
significant effect to the flavor score of jelly candy.
According to Table 4., panelist gives lower score on
higher extract concentration due to addition of
higher amount extract affects the flavor of candy
negatively. Based on the result, addition of krokot
leaf extract to jelly candy up to 2.0% shows no
significant difference to the aroma and chewiness
score.
Hedonic sensory evaluation is done by
determining the likeability of panelist towards color,
flavor, and overall. The result is expressed in Table
5.
Table 5: Effect of Krokot Leaf Extract to hedonic of jelly
candy.
Concentration Color Flavor Overall
0.5% 5.08±0.99
b
5.47±1.06
c
5.29±0.87
c
1% 4.23±1.20
a
4.73±1.27
b
4.74±1.16
b
1.5% 4.14±1.32
a
4.37±1.53
ab
4.44±1.28
ab
2% 3.90±1.44
a
4.11±1.67
a
4.29±1.54
a
3.9 Effect of Krokot Leaf Extract
Concentration on Proximate
Analysis of Produced Jelly Candy
The chosen jelly candy is the one with 0.5% krokot
leaf extract. The proximate analysis of the chosen
jelly candi is shown on Table 6. Based on the
identification result of jelly candy with chosen
krokot leaf extract using GC-MS, it is shown that the
jelly candy contains alpha-linolenic, which is an
omega-3 fatty acid. The total component analysis of
jelly candy is shown on Table 7.
Table 6: Effect of Krokot Leaf Extract to scoring of jelly
candy.
Composition Result (%)
Moisture Content 46.49±0.30
Ash Content 0.87±0.00
Fat 1.59±0.01
Protein 0.43±0.00
Carbohydrate (by difference) 50.72±0.13
Table 7: Effect of Krokot Leaf Extract to scoring of jelly
candy.
Sample Retention Time Qual
Jelly candy with
0.5% krokot leaf
extract
13,636 minutes 99%
4 CONCLUSIONS
Proximate analysis shows krokot leaf powder has
moisture content of 8.44%, which will be extracted
using by maceration on the next stage. Highest
extract yield is obtained by using food grade ethanol
as solvent, with 8.30% yield. From GC-MS analysis,
it is shown that extract using food-grade solvents
(ethanol, ethyl acetate, hexane) contains alpha-
linolenic fatty acid. Addition of krokot leaf extract
decreases the lightness of jelly candy produced.
While the texture of jelly candy produced is
improved, the chewiness is not affected. The °Hue
value is not affected by the increase of extract
concentration. The jelly candy with 0.5% krokot leaf
extract is chosen based on panelist acceptance with
overall acceptance score of 5.29. The chosen jelly
candy has color of yellowish green with °Hue value
of 91.54%. The characteristic of chosen jelly candy
produced has total soluble solids of 34.68 °Brix,
46.49% moisture content, 1.59% fat content, 0.43%
protein content, 0.87% ash content, and 50.72%
carbohydrate content. Based on GC-MS result, the
chosen jelly candy contains alpha-linolenic acid with
retention time of 13.636 minutes and 99% qual.
REFERENCES
Abdolshahi, A., Majd, M., Rad, J., Taheri, M., Shabani, A.
and Teixeira da Silva, J. (2013). Choice of solvent
extraction technique affects fatty acid composition of
pistachio (Pistacia vera L.) oil. Journal of Food
Science and Technology, 52(4), pp.2422-2427.
Adiyasa, I., Wrasiati, N. and Wartini, N. (2015). Efektivitas
Jenis Pelarut dan Lama Ekstraksi Terhadap
Karakteristik Concrete Minyak Atsiri Kulit Jeruk
Mandarin (Citrus reticulata) [Effectivity of Solvent
Type and Extraction Time to the Concrete
Characteristic of Mandarin Orange (Citrus reticulata)
peel essential oil]. Jurnal Rekayasa dan Manajemen
Agroindustri, 3(4), [online] pp.21-29. Available at:
https://ojs.unud.ac.id/index.php/jtip/article/view/16929.
Atmaka, W., Nurhartadi, E. and Karim, M. (2013).
Pengaruh penggunaan campuran karaginan dan konjak
terhadap karakteristik permen jeli temulawak
(Curcuma xanthorrhiza Roxb.) [Effect of carrageenan
16th AFC 2019 - ASEAN Food Conference
88

and konjac mixture towards curcuma (Curcuma
xanthorrhiza Roxb.) jelly candy]. Jurnal Teknosains
Pangan, [online] 2(2), pp.66-74. Available at:
https://jurnal.uns.ac.id/teknosains-pangan/article/view/
4380
Azizah, N. (2012). Pembuatan Permen Jelly dari
Karaginan dan Konjak dengan Aplikasi Prebiotik
Xilo-Oligosakarida [Production of Carrageenan and
Konjac Based Jelly with application of Xylo-
Oligosaccharide Prebiotic]. Bogor: Institut Pertanian
Bogor
Calviello, G. and Serini, S. (2010). Dietary Omega-3
Polyunstaturated Fatty Acids and Cancer. New York:
Springer Science+Business Media B.V.
Charoen, R., Savedboworn, W., Phuditcharnchnakun, S.
and Khuntaweetap, T. (2015). Development of
Antioxidant Gummy Jelly Candy Supplemented with
Psidium guajava Leaf Extract. KMUTNB International
Journal of Applied Science and Technology, 8(2),
pp.145-151.
Delgado, P. and Bañón, S. (2014). Determining the
minimum drying time of gummy confections based on
their mechanical properties. CyTA - Journal of Food,
13(3), pp.329-335.
Diana, F. (2012). Omega 3. Jurnal Kesehatan Masyarakat,
2(6).
Farikha, I., Anam, C. and Widowati, E. (2013). Pengaruh
jenis dan konsentrasi bahan penstabil terhadap
karakteristik fisikoimmia sari buah naga merah
(Hylocereus polyrhizus) selama penyimpanan [Effect
of Type and Concentration of Stabilizer towards
Physicochemical Characteristic of Red Dragonfruit
(Hylocereus polyrhizus) Juice during Storage]. Jurnal
Teknosains Pangan, 2(1), pp.30-38.
Hasyim, H. and Rahim, A. (2015). Karakteristik Fisik
Kimia dan Organoleptik Permen Jelly dari Sari Buah
Srikaya pada Variasi Konsentrasi Agar-Agar
[Physical, Chemical, and Organoleptic Characteristic
of Soursop Juice Jelly Candy with Carying Agar
Concentration]. . Abrotekbis, 3(4), pp.110-116
Hunterlab. (2012). Measuring Color using Hunter L, a, b
versus CIE 1976 L*a*b*. [online] Available at:
http://www.hunterlab.com [Accessed 21 Nov. 2017].
Imeson, A. (2010). Food Stabilisers, Thickeners and
Gelling Agents. New Delhi: Blackwell Publishing Ltd.
Kaveh, M., Eidi, A., Nemati, A. and Boskabady, M.
(2017). The Extract of Portulaca oleracea and Its
Constituent, Alpha Linolenic Acid Affects Serum
Oxidant Levels and Inflammatory Cells in Sensitized
Rats. Iran J Allergy Asthma Immunol., 16(3), pp.256-
270. Available at: http://ijaai.tums.ac.ir/index.php/
ijaai/article/view/1078.
Kemp, S., Hollowood, T. and Hort, J. (2009). Sensory
Evaluation a Practical Handbook. United Kingdom:
Wiley-Blackwell.
Koswara, S. (2009). Teknologi Pembuatan Permen
[Confectionary Processing Technology].
Lawless, H. and Heymann, H. (2010). Sensory Evaluation
of Food. New York: Springer.
MacDougall, D. (2002). Colour in Food. England:
Woodhead Publishing.
Nielsen, S. (2010). Food Analysis Laboratory Manual.
20th ed. New York: Springer Science+Business Media
LLC.
Octaviana, P., Purwijantiningsih, L. and Pranata, S.
(2013). Kualitas Permen Jelly dari Albedo Kulit Jeruk
Bali (Citrus grandis L. Osbeck) dan Rosela (Hibiscus
sabdariffa L.) dengan Penambahan Sorbitol [Quality
of Grapefruit Albedo (Citrus grandis L. Osbeck) and
Roselle (Hibiscus sabdariffa L.) Jelly Candies with the
Addition of Sorbitol]. Jurnal Biologi, [online] pp.1-12.
Available at: http://e-journal.uajy.ac.id/id/eprint/4386.
Official Methods of Analysis of AOAC International.
(2005). 15th ed. Washington, D.C.: The Association of
Official Analytical Chemists, p.212.
Purwaningtyas, H., Suhartatik, N. and Mustofa, A. (2017).
Formulasi Permen Jelly Ekstrak Daun Sirih (Piper
betle L.) dan Daun Suji (Pleomele angustofolia)
[Formulation of Jelly from Candy Betel (Piper betle
L.) – Suji (Pleomele angustofolia) Leaf Extract].
Jurnal Teknologi dan Industri Pangan, [online] 2(1),
pp.25-30. Available at:
http://ejurnal.unisri.ac.id/index.php/jtpr/article/view/1
532.
Puspitasari, A. and Proyogo, L. (2017). Perbandingan
Metode Ekstraksi Maserasi dan Sokhletasi terhadap
Kadar Fenolik Total Ekstrak Etanol Daun Kersen
(Mutingia calabura) [Comparison Between
Maceration and Soxhlation Extraction Method
Towards Total Phenolic Content of Kersen (Mutingia
calabura) Leaf Ethanol Extract]. Jurnal Ilmiah
Cendekia Eksakta, [online] 2(1). Available at:
https://www.publikasiilmiah.unwahas.ac.id/index.php/
CE/article/view/1791.
Rahmawati, P. and Adi, A. (2016). Daya Terima dan Zat
Gizi Permen Jeli dengan Penambahan Bubuk Daun
Kelor (Moringa oleifera) [Acceptance and Nutritional
Value of Jelly Candy with the Addition of Moringa
(Moringa oleifera) Leaf Powder]. Media Gizi
Indonesia, [online] 11(1), pp.86-93. Available at:
https://e-journal.unair.ac.id/MGI/article/view/4413.
Rahmi, S., Tafzi, F. and AnggrainiQ, S. (2012). Pengaruh
Penambahan Gelatin Terhadap Pembuatan Permen
Jelly Dari Bunga Rosella (Hibiscus sabdariffa Linn)
[Effect of Gelatin Addition Towards the making of
Jelly Candy from Rosella (Hibiscus sabdariffa Linn)
Flower]. Jurnal Penelitian Universitas Jambi, 14(1),
pp.37-44.
Riaz, M. and Chaudry, M. (2004). Handbook of halal food
production
. Washington, D.C: CRC Press LCC.
Schmid, M., Guihéneuf, F. and Stengel, D. (2016).
Evaluation of food grade solvents for lipid extraction
and impact of storage temperature on fatty acid
composition of edible seaweeds Laminaria digitata
(Phaeophyceae) and Palmaria palmata (Rhodophyta).
Food Chemistry, 208, pp.161-168.
Septiani, I., Basito, B. and Widowati, E. (2013). Pengaruh
Konsentrasi Agar-agar dan Karagenan terhadap
Karakteristik Fisik, Kimia, dan Sensori Selai
Purslane (Portulaca Oleracea L.) Leaves Extract Addition in Jelly Candy Making
89

Lembaran Jambu Biji Merah (Psidium guajava L.)
[Effect of Agar-agar and Carrageenan towards its
Physical, Chemical, and Sensory Characteristic of
Guava (Psidium guajava L.) Sheeted Jam]. Jurnal
Teknologi Hasil Pertanian, 4(1), pp.27-35.
Sperber, W. and Doyle, M. (2009). Compendium of
Microbiological Spoilage of Foods and Beverages.
London: Springer Science+Business Media, LLC.
Subaryono and Utomo, B. (2006). The Use of
Carrageenan–Konjac in Jelly Candy Production.
Jurnal pascapanen dan bioteknologi kelautan dan
perikanan, 1(1), pp.19-26.
Sugiharto, R., Setyani, S. and Rikafilanti, N. (2015). Efek
fortifikasi minyak ikan terhadap kadar omega-3 dan
sifat sensori roti tawar selama penyimpanan [Effect of
fish oil fortification to the level of omega-3 and
sensory of bread during storage]. Jurnal Teknologi
Industri & Hasil Pertanian, 20(1), pp.38-50.
Uddin, M., Juraimi, A., Hossain, M., Nahar, M., Ali, M.
and Rahman, M. (2014). Purslane Weed (Portulaca
oleracea): A Prospective Plant Source of Nutrition,
Omega-3 Fatty Acid, and Antioxidant Attributes. The
Scientific World Journal, 2014, pp.1-6.
U.S Departemen of Agriculture National Nutrient Data
(USDA). (2015). USDA National Nutrient Data Base
for Standard Reference “Beta Carotene”.
Williams, P. and Phillips, G. (2004). Gums and Stabilizer
for Food Industry 12. UK: The Royal Society of
Chemistry.
Williams, P. and Phillips, G. (2009). Handbook of
Hydrocolloids, 2nd edition. UK: Woodhead
Publishing Limited
Yessoufou, A., Nekoua, M., Gbankoto, A., Mashalla, Y.
and Moutairou, K. (2015). Beneficial Effects of
Omega-3 Polyunsaturated Fatty Acids in Gestational
Diabetes: Consequences in Macrosomia and
Adulthood Obesity. Journal of Diabetes Research,
2015, pp.1-11.
Zhou, Y., Xin, H., Rahman, K., Wang, S., Peng, C. and
Zhang, H. (2015). Portulaca oleracea L: A Review of
Phytochemistry and Pharmacological Effects. BioMed
research international.
16th AFC 2019 - ASEAN Food Conference
90
