
Characterization of Edible Film Made of Pectin from Nutmeg and
Palmitic Acid
Payung Layuk, Meivie Lintang and Stevie Karouw
North Sulawesi Assessment Institute for Agricultural Technology, Jl. Kalasey-Minanga, Malalayang Dua,
Kec. Malalayang, Manado, North Sulawesi, Indonesia
Keywords: Nutmeg, Pectin, Edible Film.
Abstract: The development of biopolymers as a packaging material is increasingly needed to reduce environmental
pollution due to the use of synthetic plastics which are biologically difficult to break down. Nutmeg contains
pectin which can be used as a base for making edible films. The aim of the study was to determine the
characterization of the edible film from the pectin of nutmeg with the addition of palmitic acid and glycerol
as a plasticizer. The results showed that the increase in the concentration of pectin and palmitic acid tended
to significantly increase the thickness of the edible film, the elongation rate and the tensile strength of the
film, but it could decrease the water vapor transmission rate and the film solubility. The best treatment was a
concentration of 30% wet pectin (w / v) and palmitic acid 0.04% (w / w) which resulted in the lowest water
vapor transmission rate of 2.39 (g / mm / m
2
hour).
1 INTRODUCTION
Packaging is one way to protect food from the
influence of internal and external factors that cause
damage. Thus the role of packaging materials is very
important.Plastic is the most widely used for
packaging material, but plastic have weaknesses such
as non-biodegradable properties, which remain in the
environment for long periods of time, they remain
threats to the environment (Krochta and Johnston
1997).
The need for packaging materials for developed
countries reaches 250 kg / per capita / year, while for
countries that are starting to develop and are poor, is
5 kg / capita / year. Approximately 30% of the total
residential solid waste is packaging material and 13%
of this amount is plastic waste. In other words, plastic
waste reaches 4% of the total residential solid waste.
The biggest demand for packaging is in the form of
flexible packaging which reaches 42% of total sales.
Followed by Paper Board Packaging 28%, Rigid
Plastic Packaging 14%, Woven Polyolefin Sack 6%,
Metal Can Packaging 5%, Glass Container Packaging
3% and others 2%. Due to the nature of plastic which
is difficult to break down naturally, its existence starts
to pollute the environment. However, plastic has the
advantages of being lightweight, strong and
economical. With these superior properties, it is
estimated that if the plastic is not replaced with other
packaging materials, it is estimated that the weight of
packaging waste will increase by 100%, the volume
will increase by 250% and the cost will increase by
250%. Therefore, it is necessary to develop other
packaging materials that have superior properties
such as plastic which is strong, lightweight and
economical as well as being biodegradable and even
edible.
In developed countries such as America, it has
responded to this challenge by developing edible
films using organic biopolymers from agricultural
products (Dumat, 1999). Edible film is a thin material
that covers a food ingredient and is safe for
consumption. Edible film serves as a barrier against
the transfer of water vapor, aroma, oxygen and gases
as well as protecting against microbial attack.
Usually, the function of edible films is enhanced by
adding antioxidants, antimicrobials, nutrients and
other food additives. The basic ingredients for
making edible films are biopolymers of agricultural
products including waste products such as protein
(corn, milk, wheat, soybeans) while carbohydrates
(starch, pectin, alginate) and fat. The use of
carbohydrates as a basic material for making edible
films is based on relatively cheap costs, abundance of
materials, and thermoplastic properties (Renata et al.,
Layuk, P., Lintang, M. and Karouw, S.
Characterization of Edible Film Made of Pectin from Nutmeg and Palmitic Acid.
DOI: 10.5220/0010565500003108
In Proceedings of the 6th Food Ingredient Asia Conference (6th FiAC 2020) - Food Science, Nutrition and Health, pages 171-177
ISBN: 978-989-758-540-1
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
171

2014). One of the sources of carbohydrates is pectin.
The results of the research by Layuk, 2001 reported
that the old nutmeg contains 21.54% pectin so that it
has the potential to be used as a base for making
edible films. The edible film made from nutmeg
pectin does not experience damage (moldy) after
being stored at room temperature due to the presence
of polyphenols in the nutmeg which can inhibit the
growth of fungi on the edible film. Edible films made
from carbohydrates such as pectin, starch and alginate
have disadvantages, including easy hydration,
expands quickly and tears easily. To overcome this, it
is necessary to add fatty acids (McHugh and Krochta
1994). Edible film requires a plasticizer. Plasticizer
can flex and prevent the brittleness of the edible film.
Glycerol is a plasticizer, which is commonly used in
making edible films (Han, 2005). Glycerol contains
relatively small hydrophilic molecules and easily
inserted between the polymer chains of the base
material. This condition causes structural
modification of the molecules making up the edible
film. Glycerol molecules will disrupt the polymer
cohesiveness of the base material by reducing
intermolecular interactions and increasing polymer
mobility thereby improving the flexibility and
extensibility of the edible film.
Permeability concerns the process of transferring
solution and diffusion when the solution moves from
one side of the film and then diffuses to the other side
of the film. The thicker the edible film produced, the
better the ability of the edible film to hold water
vapor. Fatty acids such as glycerol and palmitic acid
have hydrophilic groups that reduce molecular
density so that they can form free space in the film
matrix which facilitates diffusion of water vapor
(Ruan et al. 1998). Several studies have combined
two types of materials to improve the quality of edible
films such as; breadfruit and chitosan (Setiani et al.,
2013), whey protein concentrate and / or with
mesquito gum / sodium alginate / caragenate
(Villagomez-Zavala, et al., 2008), pectin and tapioca
(Layuk, 2001), sago starch and carrageenan
(Anggraini 2012). Nutmeg pectin and sago starch
(Layuk, et al, 2019), lindur fruit starch with
carrageenan (Jacoeb, 2014), soybean and tapioca
extract (Sinaga et al, 2013), Dangke and Agar (Fatma,
et al, 2015), starch sweet potato with glycerol (2018)
and nutmeg pectin and sago starch (Layuk, et al,
2019).
The research aimed to develop nutmeg pectin as a
raw material for making edible films with the addition
of plasticizers (palmitic acid) in various
concentrations and their effects on the characteristics
of the films produced.
2 MATERIALS AND METHODS
2.1 Materials and Tools
The materials used in the study were nutmeg, palmitic
acid, glycerol, calcium chloride (CaCl2), distilled
water, HCl and alcohol. The tools used is a digital
scale, beaker glass, measuring cup, hot plate stirrer,
magnetic stirrer, thermometer, glass plate measuring
8 x 7 x 2 cm, oen, Ioyid Instrument, micrometer and
other auxiliary equipment.
2.2 Research Methods
The research was carried out at the Laboratory of the
North Sulawesi Agricultural Technology Study
Center and the Laboratory of the Faculty of
Agricultural Technology, UGM Yogyakarta, from
March to December 2019. The stages of the research
included the isolation of pectin, making edible film
and the characteristics of the edible film.
2.3 Pectin Isolation (Layuk 2001)
Nutmeg pectin is obtained through several stages.
Ripe nutmeg is washed and cut in half to separate the
seeds and pulp. The pulp of the nutmeg is then cut
into 2 x 2 cm cubes. Then blanched for 5 minutes in
boiling water to activate the enzyme. Then dried in an
oven at 50 C for 8-12 hours until the moisture content
reaches 10-11%. Dried nutmeg is ground with a
fineness level of 50 mesh. Then the nutmeg powder
was extracted using HCl pH 2.0 at a temperature of
80 C for 2 hours. The schematic of pectin extraction
and isolation is shown in Figure 1.
2.4 Edible Film Making
Nutmeg pectin with concentrations of 10% (A1), 20%
(A2), 30% (A3) and 40% (A4) w / v and palmitic acid
0% (B0), 0.02% (B1), 0, 04% (B2) and 0.06% w / w
pectin. The solution is made by adding pectin to 100
ml of distilled water which already contains 2% sago
starch. , then heated at a temperature of 85 C for 15
minutes while stirring with a magnetic stirrer. After
that the temperature was lowered to 40 C, then added
palmitic acid and glycerol. The heating was continued
again until the temperature was 85 C while stirring
with magnetic stirrer for 10 minutes until the solution
was homogeneous. The solution is then poured into a
glass plate mold measuring 8 x 7 x 2 cm (length x
width x thickness), dried at 50 C for 10-12 hours
(Figure 2). The film was then removed from the mold
and stored in a plastic container filled with silica gel
6th FiAC 2020 - The Food Ingredient Asia Conference (FiAC)
172

.
Figure 1: Pectin isolation.
for 12 hours. Relative humidity in a plastic container
is around 40-50%. The film is then cut into pieces
according to the parameters to be tested. Tensile
strength and elongation tests were 3 x10cm, for
WVTR a circle was made with a diameter of 7 cm.
The solubility is 2 cm x 2 cm. Before taking
measurements, the film was conditioned for 24 hours
in a plastic container containing silica gel.
The experiment used was factorial completely
randomized with three replications (Gomez and
Gomez, 1995)
2.5 Data Analysis and Collection
Procedure
Measurement of water content, ash content and
protein content (AOAC, 1984), methoxyl and
polygalaruronic concentrations (Rangana, 1977).
Testing thickness, tensile strength and elongation
(Gontard, et al, 1992) tensile strength and elongation
were measured using a Universal Testing Machine
(LIoyd Instrument), while film thickness was
measured using a micrometer ( accuracy 0.001 mm).
2.6 Film Solubility (Gontard et al, 1992)
The percentage of film solubility was determined by
heating the film at 100 C for 24 hours with two pieces
of edible film with a size of 2 x 2 cm. Weighed then
immersed in 50 ml of water containing 0.02% sodium
azide.
The immersed edible film was stored at 20 C for
24 hours. Then the film was taken and dried at 100 C
for 24 hours. To determine the solubility of the edible
film, it was calculated by subtracting the initial weight
minus the insoluble dry weight multiplied by 100%.
Figure 2: Edible film making.
2.7 Water Vapor Transmission Rate
WVTR Testing (ASTM, 1980)
The water vapor transmission rate (WVTR) using the
gravimetric method was determined using the ASTM
procedure (1980). The film to be tested is glued to a
bowl made of acrylic with an outer diameter of 8 cm,
an inner diameter of 7 cm and a thickness of 2 cm,
then filled with 50 ml of water. The acrylic bowl is
then stored in a jar containing silica gel. Relative
humidity 20%. The water vapor that diffuses through
the film will be absorbed by the silica gel, the amount
of which can be found by calculating the reduced
weight of the bowl filled with water at the time of
measurement. Weighing was done at intervals,
1,2,3,4,5,6 and 7 hours. All tests were carried out
twice. Changes in bowl weight and time are then
plotted on a graph where the y-axis is the weight of
the plate (g) when weighed and the x-axis is time
(from this graph a regression line equation will be
generated where the resulting slope is the rate of
weight gain per unit (g / hour). WVTR is calculated
Characterization of Edible Film Made of Pectin from Nutmeg and Palmitic Acid
173
by dividing the slope by the area of the film, so that
the WVTR unit is g / m2h.
3 RESULTS AND DISCUSSION
3.1 Nutmeg Pectin Identification
The results of the composition analysis and
identification of pectin are as shown in Table 1. The
results showed that the pectin obtained was high
methoxyl pectin. Towle and Chistinsesn (1973) stated
that pectin which has a methoxyl count of 7-14% is a
high methoxyl pectin. Meanwhile, if the methoxyl
content is 0 -7%, it is a low methoxyl pectin. The
galacturonic content obtained was 84.18% and
compared to the commercial pectin the level was
83.78%, it can be said that the pectin produced has a
level of purity. This is supported by the low ash
content of 1.49%. High methoxyl and
polygalacturonic content and low ash content indicate
the level of purity of pectin and the factors that play a
role in the gel formation of a pectin. The presence of
protein content can also support the success of
nutmeg pectin as a basic material for making edible
films.
Table 1: Nutmeg pectin composition with commercial
pectin.
Composition Nutmeg
p
ectin *
Commercial
p
ectin **)
Yield (%)
Water content (%)
Ash content (%)
Protein Content (%)
Methoxyl content
(%)
Polygalacturunoate
content
(
%
)
21,54
8,95
1,49
4,37
11,48
84.17
-
11,05
3,42
1,95
11,42
83.78
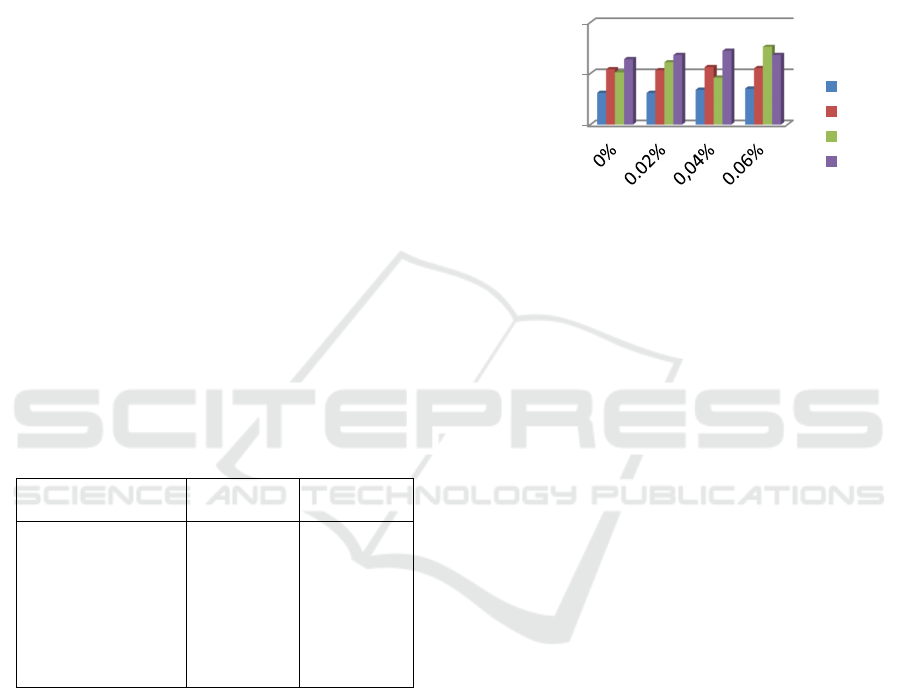
3.2 Film Thickness
The thickness of the films obtained ranged from 0.03
to 0.07 mm. The highest thickness was obtained at 3%
pectin concentration and 0.6% palmitic acid (A3B3).
In Figure 3, it can be seen that the higher the
concentration of pectin and palmitic acid, the thicker
the film produced. This occurs because the increasing
number of film-forming components, resulting in the
thicker film produced. The thickness of the film is
influenced by the amount of solids in the film forming
solution and the thickness of the mold. With the
addition of glycerol as a plasticizer, it can reduce the
thickness and force density between pectin molecules
so that the chain movement is good and the resulting
film is more flexible and smoother. This is possible
because glycerol is a small hydrophilic molecule that
can easily enter between pectin chains and form
bonds (Gontard et al, 1992, Layuk et al, 2019). The
same thing was reported by Sinaga et al, (2013) that
the addition of plasticizers such as glycerol has an
effect on the thickness of the edible film, elongation
and tensile strength of the film.
Figure 3: Edible film thickness at various concentrations of
pectin and palmitic acid.
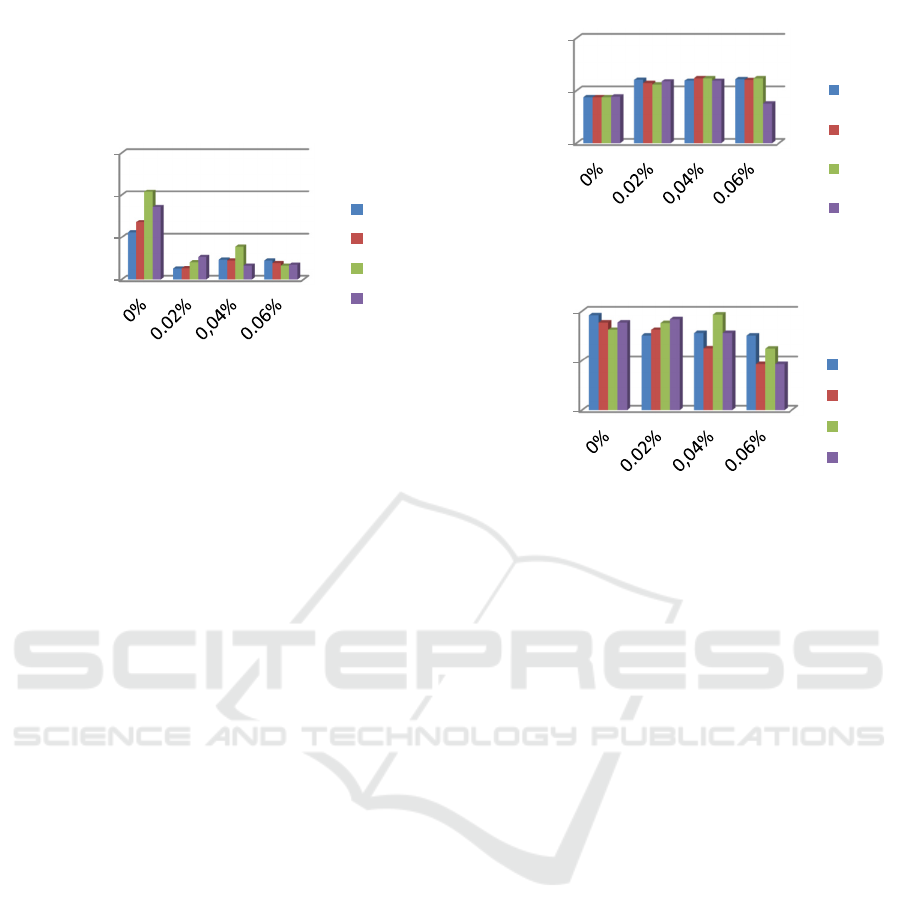
3.3 Elongation
Film elongation is a mechanical property related to
the chemical structure of the film. Film elongation is
the change in the maximum elongation experienced
by the film during the tensile strength test at the time
the film is torn (Hay, 1968). According to Gontard
and Gilbert (1993), the mechanical properties of films
depend on the type of material, especially the
structural cohesion properties. This property is a
result of the polymer's ability to form strong
molecular bonds. The results of variance can be seen
that there is a very significant effect between the
pectin concentration on the percentage of film
elongation. The palmitic acid concentration of 0.2%
and without the addition of palmitic acid had a high
elongation compared to other treatments, both at
10%, 20% and 30% pectin concentrations. This is
because palmitic acid has anti-plasticizer properties
against pectin edible film. Gontard and Gulbert
(1993) said that fatty acids have anti-plasticizing
properties in films made from gluten. The same thing
was reported (Layuk, 2001 and Layuk et al, 2019) that
the anti-plasticizing properties of palmitic acid in
pectin were caused by the formation of a
galacturonic-fatty acid complex which will add to the
three-dimensional network integrity of the polymer
film, thereby reducing the elongation rate of the film.
Meanwhile, Min Lai and Hucy (1997) reported that
palmitic acid can act as a plasticizer that can change
the mechanical characteristics of edible films. The
addition of palmitic acid will be able to increase the
0
0,05
0,1
Film Thickness (mm)
Palmitic acid concentration
Pectin
concentration
10%
20%
30%
40%
6th FiAC 2020 - The Food Ingredient Asia Conference (FiAC)
174
0
5
10
15
Film elongation (%)
Palmitic acid concentration
Pectin
concentration
10%
20%
30%
40%
0
20
40
Tensile Strength (KPa)
Palmitic acid concentration
Pectin
concentration
10%
20%
30%
40%
90
92
94
Film solubility (%)
Palmitic acid concentration
Pectin
concentration
10%
20%
30%
40%
tensile strength of the film breaking. When it reaches
a critical point, the addition of palmitic acid (Park et
al., 2004) and glycerol (Fantasari et al) will decrease
the elongation and tensile strength of the film.
Figure 4: Edible film extension at various concentrations of
pectin and palmitic acid.
3.4 Tensile Strength
Tensile Strength is a mechanical property related to
the chemical structure of the film. Tensile strength is
defined as the maximum tensile stress that the sample
can accept. The mechanical properties of the edible
film depend on the film-forming material, especially
the structural cohesion properties.
This property is a
result of the polymer's ability to form strong
molecular bonds Gontard and Gulbert (1993). In
Figure 5, it can be seen that the higher the
concentration of pectin and palmitic acid the higher
the resulting tensile strength. The highest tensile
strength occurred in the treatment with pectin
concentration of 30% and palmitic acid 0.6%, which
is 8.71 kPa. This is because the galacturonic-palmitic
acid complex can add to the polymer integration of
the edible film so that the resulting tensile strength is
higher. The addition of palmitic acid and glycerol can
improve the mechanical properties of the film where
the film is stronger, more compact and flexible. The
addition of palmitic acid also increases the number of
carbons and functional groups in the matrix chain
thereby increasing the tensile strength and elongation
(Donhowe and Fennema 1994).
3.5 Edible Film Solubility
Solubility in a solution is one of the determining
factors in choosing edible film as a packaging
material. Figure 6 shows that the solubility of the film
decreases as more palmitic acid is added. This is due
to palmitic acid which has a high C chain (C16)
making it difficult to dissolve in water. According to
Koelsch and Labuza (1992), palmitic acid can dissolve
in polar and non-polar organic solvents. The ability to
Figure 5: Tensile strength of edible films at various
concentrations of pectin and palmitic acid.
Figure 6: Edible film solubility at various concentrations of
pectin and palmitic acid.
dissolve in water is influenced by the C chain. The
longer the C chain of fatty acids, the more difficult
their solubility in water is.
3.6 Water Vapor Transmission Rate of
Film (WVTR)
The ability of the film to withstand the water vapor
transmission rate can be seen in Figure 7. The
resulting water vapor transmission rate ranges from
2.39 g / mm / m2 / hour - 5.10 g / mm / m2 / hour.
The highest WVTR was obtained at treatment
concentrations of pectin 40% and palmitic acid 006%.
The high WVTR is due to the increasing number of
hydrophilic components in the film which causes
water absorption on the film and increases the weight
of the film during the WVTR measurement. This
proves that biodegradable films require hydrophobic
materials to reduce the rate of water vapor
transmission through the film. While the lowest was
at the pectin concentration of 30% and palmitic acid
0.4%. According to Hui (2006), palmitic acid can act
as a plasticizer that can change the mechanical
characteristics of edible film, which can increase
hydrogen bonding thereby reducing water vapor
transmission rates. However, when it reaches a
critical point the addition of palmitic acid will reduce
hydrogen bonding and increase the intermolecular
space which will increase the flexibility of the film
and provide a cavity that allows diffusion of penetrant
Characterization of Edible Film Made of Pectin from Nutmeg and Palmitic Acid
175

molecules (Layuk 2001 and Slade and Levine, 1991).
This is also due to the presence of glycerol which
causes the speed of water vapor and gas transmission
through the film to increase (Gontar et al., 1983) and
due to reduced molecular density so that free space is
formed in the film matrix so as to facilitate the
diffusion of water and gas. The same thing was found
in this study where the palmitic acid concentration of
0.6% both at 10%, 20% and 30% pectin
concentration, resulted in increased WVTR and the
resulting film surface was rather rough.
Figure 7: Water vapor Transmission Rate Edible film at
various concentrations of pectin and palmitic acid.
4 CONCLUSIONS
Nutmeg pectin can be used as material for
making edible films.
Increasing the concentration of pectin and
palmitic acid can increase the thickness,
elongation and tensile strength of the film, but
can reduce WVTR and film solubility.
The best treatment was pectin concentration of
30% and palmitic acid 0.4% where the smallest
water vapor transmission rate was 2.39g / mm /
m2 / hour.
REFERENCES
AOAC. 1984. Official Method of Analysis of Association
of Official Analytical Chemists, 14thEd. AOAC Inc.,
Arlington,Virginia.
ASTM. 1980. Standart Test Methods for Water Vapor
Transmission Of Materials. ASTM Book of Standards
Anggraini 2012. Characteristics of Edible Film from Sago
Starch with the Addition of Glycerol and Carrageenan.
Journal of Food and Agroindustry 1: 12-1
Bourtoom T. 2008. Review article edible films and
coatings: characteristics and properties. Journal
International Food Research 15(3):237-248.
Donhowe,G. and O. Fennema. 1994. Edible film and
Coting: Characteristics Formation, Definitions and
Testing Methods: Krochta, J.M., E.A.
Baldwin, and M.O.Nisperos-Carriedo (eds). Edible
Coatings and Films to Improve Food Quality.
Tecnomic Publ.Co.,Inc.,Lancaster, USA.
Dumat, 1996. Making edible films from a mixture of rubber
seed protein with casein. Postgraduate Thesis.Master
Program in Food and Science Technology. Faculty of
Agricultural Technology UGM.
Fatnasari, A. K. A. Nocianitri and I Putu Suparthana. The
Effect of Glycerol Concentration on The Characteristic
Edible Film Sweet Potato Starch (Ipomoea batatas L.).
Scientific Journal Of Food Technology Vol. 5, No.1, 27
- 35, March 2018.
Fatma, Ratmawati Malaka and Muhammad Taufik.2015.
Characteristics of Edible Film Made from Dangle
Whey and Agar Using Different Percentage of
Glycerol. JITP Vol. 4 No. 2, Page 63-69, July 2015
Fennema O, Donhowe IG, Kester JJ. 1994. Lipid type and
location of the relative humidity gradient influence on
the barrier properties of lipid to water vapor. Journal
of Food Engineering 22(1):225-239
Gontar, N. Guilbert and J.L. Cug. 1992. Water and
Glycerol as Plasticizer affect mechanical and water
vapor barrier properties of edible film wheat gluten
film. J. Food Sci: 58(1): 206-210
Gontard, N. and Gulbert,S. 1993. Bio Packaging.
Technology and properties of edible film and
biodegradable material of agricultural origin. In M.
Methiouthi. Food packaging and preservation. The AVI
Publ.Inc. Westport Connecticut.
Han, 2005. Innovations in Food Packaging. Department of
Food Science University of Manitoba Wiminpeg,
Manitoba Canada.
Hay,P.M. 1968. Properties and Methods of Identification of
Commercial Films. In O.J. (ed) .The Science and
Technology of Polymer Film, London.
Hui, Y. H. 2006. Handbook of Food Science, Technology,
And, Engineering Volume I. Crc Press. USA.
Jacoeb M. A. Roni Nugraha, Siluh Putu Sri Dia Utari. 2014.
Edible Film from Lindur Fruit Starch with Addition of
Glycerol and Carrageenan. JPHPI 2014, Volume 17
No 1.
Koelsch C.M. and Labuza, T.P. (1992). Functional
physical and morphological properties of methyl
cellulose and fatty acid-based edible barriers. Lebensm
Wiss U-Technol 25:404-411.
Krochta JM and Johnston CDM. 1997. Edible and
Biodegradable Polymer Film. Journal of Food
Technology 52(2): 1-20.
Layuk, P. 2001. Characterization of Nutmeg Pectin
Composite Edible Film with Tapioca.
PostgraduateThesis.Master Program in Food and
Science Technology. Faculty of Agricultural
Technology UGM
Layuk P . Sondakh J. Marietje P. Characteristics and
Permeability Properties of Sago Starch Edible Film.
AGRITEKNO, Journal of Agricultural Technology
Online Version: http://ojs3.unpatti.ac.id/index.php/
0
5
10
Water Vapor
Transmission Rate (%)
Palmitic acid concentration
Pectin
concentration
10%
20%
30%
40%
6th FiAC 2020 - The Food Ingredient Asia Conference (FiAC)
176

agritekno Vol. 8, No. 2, 34-41, Th. 2019 DOI:
10.30598/jagritekno.2019.8.2.34 ISSN 2302-9218
McHugh TH, Krochta JM. 1994. Sorbitol vs Glycerol
plasticized whey protein edible films: integrated oxygen
permeability and tensile strength property evaluation.
Journal of Agricultural and Food Chemistry 42(4):841-
845.
Min Lai an Huey 1997. Properties of microstructure of
sheets plasticized with palmitic acid. J. Cereal
Chemiestry 42(2).
Park, D.P., Sung, J.H., Choi, H.J. and Jhon, M.S. (2004).
Electroresponsive characteristics of highly substituted
phosphate starch. Journal of Material Science 39:
60836086.
Park JW, Testin, RF, Vergano DJ, Park KJ, Weller CL,
1996. Application of Laminated Edible Film to Potato
Chip Packaging. Journal of Food Science 61(4): 66-76
Rangana S. 1977. Manual of Analysis of Fruit and
Vegetable Product. Tat MC. Grow Hill Publishing
Company Limited New Delhi.
Renata C., dos Reis., I. A. Devilla, G. H. H. Oliveira, P. C.
Corrêa, D. P. R. Ascheri, A. B. M. Souzay and A. C. O.
Servulo. 2014. Mechanical Properties, Permeability
and Solubility Of Films Composed of Yam Starch and
Glycerol. INVERCIENCIA, 39: 410415.
Ruan RR, Xu L, Chen PL. 1998. Water Vapor Permeability
and Tensile Strength of Cellulose-Based Composite
Edible Film Applied Engineering In Agriculture
14(4):411-413.
Setiani, W., T. Sudiarti, L. Rahmidar. 2013. Preparation
and characteristics of edible films from polyglend
breadfruit starch-chitosan. Valensi, 3(2): 100-10.
Sinaga L.L., Melisa S. R .S, Mersi S. S. 2013.
Characteristics of Edible Film From Soybean Extract
With The Addition Of Tapioca Flour And Glycerol As
Food Packaging Materials USU's Chemical
Engineering Journal, Vol. 2, No. 4 (2013)
Slade and Levine, 1991. Beyond water activity: Recent
advances on an alternative approach to the assessment
of food quality and safety. Food Sec. 61(1): 192-194.
Towle, G.G. and Chistinsen, O. 1973. Pectin, in
R.L.Wister Industrial GUM. Academic press New
York.
Villagomez-Zavala, D. L., C. Gomez-Corona, E. S.
M.Matinez and J. P. P. Orozco. 2008. Comparative
study the mechanical properties of edible films made
from single and blended hydrophilic biopolymer
matrices. Revista Mexicana de Ingenieria Quimica,
7(3): 263-273.
Characterization of Edible Film Made of Pectin from Nutmeg and Palmitic Acid
177
