
Electroencephalography-based Motor Hotspot Detection
Ga-Young Choi
1a
, Chang-Hee Han
2b
, Hyunmi Lim
3c
, Jeonghun Ku
3d
, Won-Seok Kim
4e
and Han-Jeong Hwang
1f
1
Department of Medical IT Convergence Engineering, Kumoh National Institute of Technology,
Gumi 39177, Republic of Korea
2
Machine Learning Group, Berlin Institute of Technology (TU Berlin), 10623 Berlin, Germany
3
Department of Biomedical Engineering, School of Medicine, Keimyung University, Republic of Korea
4
Department of Rehabilitation Medicine, Seoul National University College of Medicine, Seoul National University
Bundang Hospital, Seongnam-si, Republic of Korea
Keywords: Neuronavigation, Hotspot, Electroencephalography, Transcranial Magnetic Stimulation.
Abstract: The motor-evoked potential (MEP) induced by transcranial magnetic stimulation (TMS) has been generally
used to identify a motor hotspot, and it has been used as a target location for transcranial electrical stimulation
(tES). However, the traditional MEP-based method needs a bulky TMS device, and it involves the empirical
judgement of an expert. In this study, we propose a machine-learning-based motor hotspot identification
method using electroencephalography (EEG) that is portably acquired in a tES device. EEG data were
measured from ten subjects while they performed a simple finger tapping task. Power spectral densities
(PSDs) were extracted from the EEG data as features, and they were used to train and test artificial neural
network (ANN). The 3D coordinate information of individual motor hotspots identified by TMS were also
used as the ground-truth motor hotspot locations in ANN, and they were compared with those estimated by
ANN. A minimum distance between the motor hotspots identified by TMS and EEG features was only 0.24
cm, demonstrating the feasibility of our proposed novel motor hotspot identification method based on EEG
features.
1 INTRODUCTION
Non-invasive brain stimulation (NIBS) is an
emerging technique that applies electrical current or
magnetic field to the scalp for the modulation of
cortical excitability (Paulus, 2000). NIBS is divided
into two types according to whether electrical current
or magnetic field is used. NIBS based on electrical
current is called transcranial electrical stimulation
(tES) that is divided into three types: i) transcranial
direct current stimulation (tDCS) (Nitsche et al.,
2000), transcranial alternating current stimulation
(tACS) (Herrmann et al., 2013), transcranial random
noise stimulation (tRNS) (Antal et al, 2016). NIBS
a
https://orcid.org/0000-0003-2209-5517
b
https://orcid.org/0000-0001-8668-3989
c
https://orcid.org/0000-0001-7074-7757
d
https://orcid.org/0000-0002-9610-0078
e
https://orcid.org/0000-0002-1199-5707
f
https://orcid.org/0000-0002-1183-1219
based on magnetic field is called transcranial
magnetic stimulation (TMS) (Wassermann et al,
2001).
TMS has been widely used to identify muscle
representations in the motor cortex as well as to
investigate corticomotor excitability. An optimal
TMS site is called as the motor hotspot, and it is
generally identified based on the TMS-induced motor
evoked potential (MEP).
The motor hotspot identified by TMS has been
used to validate the feasibility of tES on corticomotor
excitability (Cabral et al, 2015). Some studies have
shown that tES is effective for motor function
rehabilitation in patients with stroke, Parkinson’s
Choi, G., Han, C., Lim, H., Ku, J., Kim, W. and Hwang, H.
Electroencephalography-based Motor Hotspot Detection.
DOI: 10.5220/0008937201950198
In Proceedings of the 13th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2020) - Volume 4: BIOSIGNALS, pages 195-198
ISBN: 978-989-758-398-8; ISSN: 2184-4305
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
195
disease, amyotrophic lateral sclerosis (ALS), and so
on (Hummel et al., 2006). Most tES studies have used
the anodal electrode attached to the motor hotspot
identified by TMS, and the cathodal electrode
attached to the contralateral motor area or
contralateral supraorbital area (Ferreira et al., 2019).
Although TMS is an ideal tool to find the motor
hotspot, a cumbersome procedure involving the
empirical judgement of an expert is required to find
the motor hotspot. Also, it is impractical to use a TMS
device for finding the motor hotspot as a target area
for tES because a TMS device is relatively bulky and
heavy. A potential alternative to TMS identifying the
motor hotspot is to use electroencephalography
(EEG) measured while performing a motor task
related to a targeted motor hotspot because EEG
provides the representation information related to
motor functions even though its spatial resolution is
relatively low as compared to TMS. Therefore, in this
study, we propose an EEG-based machine-learning
approach to identify an individual motor hotspot that
is used as a target location for tES.
2 METHODS
2.1 Subjects
Ten right-handed subjects (five females and five
males; 25.3 ± 1.36 years) were recruited for this
study. They have no history of psychiatric diseases
that might affect research results. They received the
information about the details of experiment
procedure, and signed an informed consent for study
participation. Appropriate compensation for their
participation was provided after the experiment. This
study was approved by the Institutional Review
Board (IRB) of Kumoh National Institute of
Technology (No. 6250), and was conducted in
accordance with the principles of the declaration of
Helsinki.
2.2 Experiment Protocols
Subjects sat on a comfortable armchair. An individual
motor hotspot was first identified using TMS. The
motor hotspot was defined as the TMS coil location
that shows a MEP with an amplitude of at least 50 μV
more than 5 out of 10 consecutive stimuli when a
minimum stimulation intensity was applied. Because
a target region of interest was the right first dorsal
interosseous (FDI) muscle in this study, MEP was
measured from the FDI muscle using Ag-AgCl
disposable electrodes while single-pulse TMS was
applied to a corresponding brain area (REMED.,
Daejeon, Korea). We searched a motor hotspot on the
contralateral motor area (around C3 based on the
international 10-20 system); the coil was held at
approximately 45 degrees with the handle facing the
rear in order for TMS to be directed perpendicular to
the brain. Individual motor hotspot locations were
represented in the 3D coordinate (x, y, and z) based
on the vertex (Cz in the 10-20 international system)
using a polhemus patriot digitizer (Polhemus Inc.,
Colchester, Vermont, USA). The 3D locations of
individual motor hotspots were used as the ground
truth, and they were compared with those identified
by EEG to verify the feasibility of our proposed EEG-
based motor hotspot identification approach.
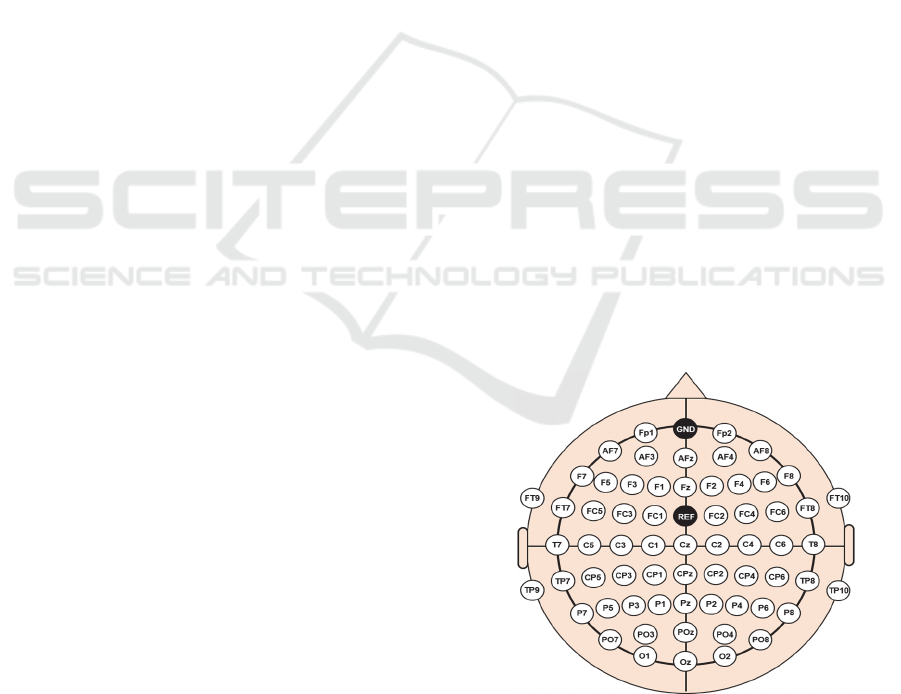
To measure motor-task-specific brain activity, 64
EEG electrodes were mounted on the scalp using the
international 10-20 system (Figure 1), and the
location of the EEG electrodes were also represented
in the 3D coordinate as that of the motor hotspot
identified by TMS-induced MEPs. The ground and
reference electrodes were attached on Fpz and FCz,
respectively. The EEG data were sampled at 1,000 Hz
using a multi-channel active electrode EEG
acquisition system (actiChamp, Brain Products
GmbH, Gilching, Germany) while each subject
performed a motor task that presses a button 30 times
using a right index finger whenever a red circle
appeared in the center of a monitor (Figure 2). The
subjects were given enough rest in the middle of the
experiment to avoid fatigue whenever they wanted. In
addition, they were instructed to remain relaxed
during the experiment without any movements to
prevent unwanted physiological artifacts.
Figure 1: Electrode positions used for recording EEG data.
BIOSIGNALS 2020 - 13th International Conference on Bio-inspired Systems and Signal Processing
196
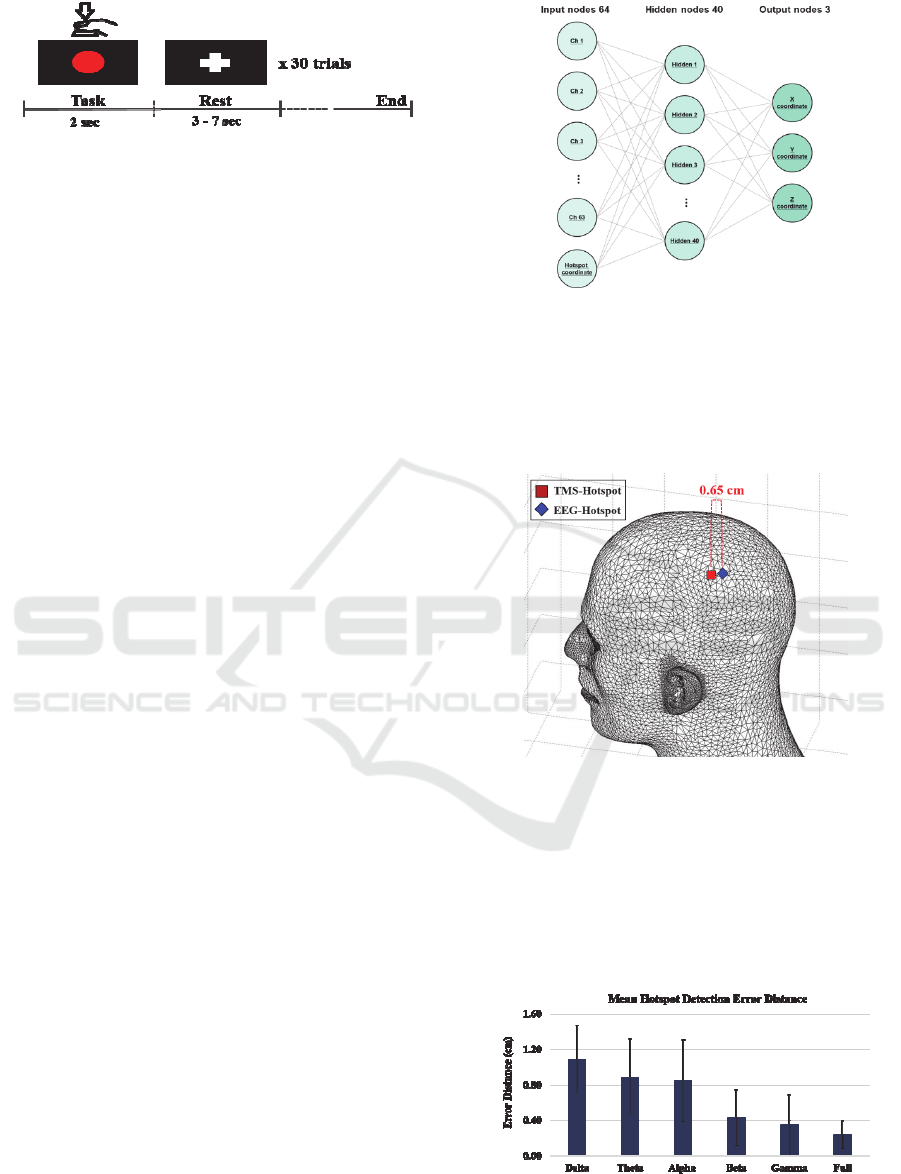
Figure 2: Experimental paradigm.
2.3 Data Analysis
EEG data were analysed using the MATLAB
(MathWorks, Natick, MA, USA). The raw EEG data
were down-sampled into 200 Hz. We applied
common average reference (CAR) and bandpass
filtering between 0.5 and 50.5 Hz (zero-phase third-
order Butterworth filter) sequentially to the down-
sampled data. We also applied multiple artifact
rejection algorithm (MARA) based on independent
component analysis (ICA) to the filtered data in order
to remove physiological artifacts.
After the EEG preprocessing, we epoched the
EEG data between -0.5 and 0.5 sec based on an onset
time when a subject pressed a button for each trial.
Power spectral density (PSD) was estimated for each
trial and each channel using the fast Fourier transform
(FFT), and the PSDs of six frequency bands were
calculated (delta:1 – 4 Hz, theta: 4 – 8 Hz, alpha: 8 –
13 Hz, beta: 13 – 30 Hz, gamma: 30 – 50 Hz, full: 1
– 50 Hz). To identify the motor hotspot based EEG, a
multi-layer feedforward artificial neural network
(ANN) was trained and tested using EEG PSD
features (Figure 3). The input labels of the ANN were
the 3D coordinate information of the motor hotspots
identified by TMS, and the outputs were their
corresponding 3D coordinate information produced
by the ANN based on the EEG PSD features. A 10-
fold cross-validation was performed with early
stopping to prevent overfitting. The distance between
the 3D coordinates of the motor hotspots identified by
TMS and EEG was calculated using Euclidean
distance, which was defined as the error distance. The
mentioned procedure was performed for each
frequency band (delta, theta, alpha, beta, and gamma)
and the whole frequency band (full) to find an optimal
EEG frequency band to extract PSD features.
3 RESULT
Figure 4 presents a representative example from one
subject, showing the 3D coordinate locations of
motor hotspots identified by TMS (red) and EEG
PSD features (blue). The detected motor hotspots are
Figure 3: Schematic diagram of an ANN model used to find
motor hotspots based on EEG features.
located in the contralateral motor area of the right
index finger, and the motor hotspot locations
identified by TMS and EEG PSD features are close to
each other (0.65 cm).
Figure 4: 3D coordinate information of motor hotspots
identified TMS (red) and EEG PSD features (blue).
Figure 5 shows the mean error distances for each
frequency band. A minimum error distance of 0.24
cm was obtained when a full band was used to extract
PSD features (1.09 ± 0.38 cm for delta, 0.89 ± 0.43
cm for theta, 0.85 ± 0.46 cm for alpha, 0.43 ± 0.31 cm
for beta, and 0.35 ± 0.34 cm for gamma).
Figure 5: Mean error distances for each frequency band.
Electroencephalography-based Motor Hotspot Detection
197

Individual error distances for each frequency band
are presented in Table 1.
Table 1: Motor hotspot error distances of each subject for
each frequency band.
Subject Delta Theta Alpha Beta Gamma Full
S1
0.82 0.92 0.31 0.13 0.03 0.11
S2
1.31 0.91 0.81 0.28 0.18 0.16
S3
0.73 0.87 0.35 0.28 0.17 0.25
S4
0.38 0.52 0.47 0.15 0.08 0.22
S5
1.22 0.71 0.48 0.33 0.16 0.09
S6
1.69 1.02 1.39 0.64 0.69 0.17
S7
1.36 0.51 1.30 1.10 0.74 0.30
S8
0.97 0.57 1.10 0.23 0.26 0.21
S9
1.03 0.89 0.72 0.39 0.15 0.21
S10
1.39 2.00 1.55 0.76 1.03 0.65
Mean
1.09 0.89 0.85 0.43 0.35 0.24
± Std.
0.38 0.43 0.46 0.31 0.34 0.16
4 CONCLUSIONS
In this study, we proposed an EEG-based novel motor
hotspot identification algorithm using machine
learning technique to provide a target location for tES
without using TMS. A minimum distance between
motor hotspots identified by TMS-induced MEP and
EEG features was 0.24 cm when using a full
frequency band information. As a tES electrode size
is generally bigger than 1 cm, it is expected that the
motor hotspot identified by EEG features could be
covered by a tES electrode with a small error
distance. However, additional tES experiments
should follow to verify the feasibility of our proposed
motor hotspot identification method based on EEG on
corticomotor excitability.
Instead of using a TMS device, an EEG device is
required to apply our proposed machine-learning-
based motor hotspot identification method. Note that
it is possible to integrate an EEG device to a tES
device with retaining its portability, and a
commercially available tES/EEG device already
exists (e.g., NeuroElectronics Starstim). Thus, we
expect that the EEG-based hotspot detection
algorithm will facilitate use of tES, in particular, for
home-based tES treatment. One limitation of our
algorithm is that TMS was used to find the 3D
coordinates of motor hotspots. Thus, we will develop
an advanced method that use the 3D coordinates of
motor hotspots identified by TMS to construct a
motor hotspot identification algorithm, after which it
uses only EEG features to find individual motor
hotpots for new subjects.
ACKNOWLEDGEMENTS
This work was supported by Ministry of Trade
Industry & Energy (MOTIE, Korea), Ministry of
Science & ICT (MSIT, Korea), and Ministry of
Health & Welfare (MOHW, Korea) under
Technology Development Program for AI-Bio-
Robot-Medicine Convergence (20001650).
REFERENCES
Paulus, W. 2011. Transcranial electrical stimulation (tES–
tDCS; tRNS, tACS) methods. Neuropsychological
rehabilitation, 21(5), 602-617.
Nitsche, M. A., & Paulus, W. 2000. Excitability changes
induced in the human motor cortex by weak transcranial
direct current stimulation. The Journal of physiology,
527(3), 633-639.
Herrmann, C. S., Rach, S., Neuling, T., & Strüber, D. 2013.
Transcranial alternating current stimulation: a review of
the underlying mechanisms and modulation of
cognitive processes. Frontiers in human neuroscience,
7, 279.
Antal, A., & Herrmann, C. S. 2016. Transcranial alternating
current and random noise stimulation: possible
mechanisms. Neural plasticity.
Wassermann, E. M., & Lisanby, S. H. (2001). Therapeutic
application of repetitive transcranial magnetic
stimulation: a review. Clinical Neurophysiology,
112(8), 1367-1377.
Cabral, M. E., Baltar, A., Borba, R., Galvão, S., Santos, L.,
Fregni, F., & Monte-Silva, K. (2015). Transcranial
direct current stimulation: before, during, or after motor
training?. Neuroreport, 26(11), 618-622.
Hummel, F. C., & Cohen, L. G. 2006. Non-invasive brain
stimulation: a new strategy to improve
neurorehabilitation after stroke?. The Lancet
Neurology, 5(8), 708-712.
Ferreira, I. S., Costa, B. T., Ramos, C. L., Lucena, P.,
Thibaut, A., & Fregni, F. 2019. Searching for the
optimal tDCS target for motor rehabilitation. Journal of
neuroengineering and rehabilitation, 16(1), 90.
BIOSIGNALS 2020 - 13th International Conference on Bio-inspired Systems and Signal Processing
198
