
A Process Reference Model for Enhanced Medication Management
Bonnie Urquhart
1a
and Waqar Haque
2b
1
Northern Health, Prince George, Canada
2
Department of Computer Science, University of Northern British Columbia, Prince George, Canada
Keywords: Business Ontology, Business Architecture, Business Process Management, Medication Management, Process
Reference Model.
Abstract: A comprehensive management approach to improve quality and safety of medication management within a
multisite health care organization is explored. The process-oriented approach integrates Business Ontology,
Business Architecture and Business Process Management to develop a reference model for medication
management. The model includes one hundred and sixty-four individual processes categorized in four process
areas and twenty-five process groups. The business artefacts and methodology used in the development of
this reference model are also presented. These artefacts were created and validated through workshops and
working group meetings. The developed methodology could be used to create a similar process architecture
in other organizations and service areas.
1 INTRODUCTION
The delivery of health care services is a complex
operation in which care providers and managers are
expected to deliver safe, high quality services within
a resource constrained system. Patient safety is of
paramount importance. The need for improvement in
the medication management processes is evident
based on the incidence of adverse medication events
reported in the literature (Martins, Giordani, &
Rozenfeld, 2014). There are a multitude of clinicians
and support staff involved in medication management
and there are numerous legislated requirements which
both contribute to the complexity of the processes.
There needs to be a shared understanding among care
providers involved in medication management
processes together with an understanding of how
medication management processes fit within the
larger health care system. Business ontology can be
used to create a shared language and identify the
relationships between business objects (Au-Yong-
Oliveria & Ferreira, 2014). Enterprise architecture
provides the structure in which the ontology can be
used to show relationships between like objects
across the organizational architectural layers or
across domains within the same architectural layer
(Hendrickx, Mahakena, & von Rosing, 2012). When
a
https://orcid.org/0000-0001-7415-1627
b
https://orcid.org/0000-0002-6921-8097
combined with Business Process Management
(BPM) (Bandra, et al., 2010) in a healthcare
environment, this yields an integrated approach to
design, evaluate and improve safety of medication
management processes. The business layer of an
enterprise architecture framework is at the core of our
proposed model. The application and technology
layers of the enterprise architecture are important
when considering the automated processes
particularly where technology such as infusion pumps
and bar code scanners are used in the processes.
(Singh)
A reference model is either a narrative or visual
conceptual representation of the recommended (best)
practices of a specific domain. A business process
reference model can be used to inform and guide the
development of a business process where no such
model previously existed or it can be used to compare
current business process to a generic reference model
which has incorporated leading or best practices
within the domain (Pajik, Indihar-Stemberger, &
Kovacic, 2012). Since developing business process
models is time consuming and expensive, reference
models can be used to shorten the time to design or
standardize process models across an organization.
The medication management process reference
model presented in this paper is a narrative
Urquhart, B. and Haque, W.
A Process Reference Model for Enhanced Medication Management.
DOI: 10.5220/0008992705470554
In Proceedings of the 13th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2020) - Volume 5: HEALTHINF, pages 547-554
ISBN: 978-989-758-398-8; ISSN: 2184-4305
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
547

representation of the processes that we believe should
be included in a process architecture for medication
management.
The challenge with producing generic business
process models is that they may be context specific;
therefore, not necessarily transferrable to other
organizations. It is possible that best practice in one
organization or industry may not translate to best
practice in another organization if there is a significant
difference in the strategy of the two organizations
(Ponsignon, Smart, & Maul, 2012). Despite these
concerns there are many examples of reference models
for business process in use and referenced in the
literature. These include SCOR – Supply Chain
Operations Reference Model (Supply Chain Council
Inc., 2012), SAP R/3 reference model and the Process
Classification Frameworks developed by the American
Productivity and Quality Council (APQC) (American
Productivity and Quality Centre (APQC), 2014).
In this paper, we present a process reference model
for medication management which consists of the
traditional core, support and management processes,
and a fourth area (Clinical Training and Professional
Development) as some included processes can be
considered to be either support, management, or both.
There were two sources of information considered for
use in validating the proposed model: the APQC
Process Classification Framework (American
Productivity and Quality Centre (APQC), 2014) and
The Supply Chain Operations Reference Model
(Supply Chain Council Inc., 2012).
2 METHODS & MATERIALS
Qualitative design, in the form of researcher facilitated
workshops and working groups, was used to develop
business artefacts that were subsequently used to
develop the process reference model. The research
was undertaken in a multi-site publicly funded
Canadian healthcare organization. The business
architecture developed by the Global University
Alliance (GUA) (Global University Alliance, 2018)
and a standard of the LEADing Practice community
(Layered Enterprise Architecture Design) was used
together with the BPM Ontology which was also
developed as part of the foundational business
ontology by GUA (von Rosing, Scheer, & von Scheel,
2014). The architecture is designed in three layers:
business layer, application layer and technology layer.
The business layer has four sub-layers, namely,
purpose and goal, business competency, business
service, and business process. The BPM ontology
includes meta-object groups that have been shown to
apply to almost any process related object and artefact
(von Rosing, Laurier, & Polovina, 2014). The sixteen
meta-object groups are: Service, Business
Competency, Purpose & Goal, Objects, Owner,
Process Flow, Roles, Process Rules, Process Security,
Application, Process Measurement, Channel, Data,
Media, Platform, and Infrastructure. The activities
involved during the design of process reference model
begin by identification of the purpose and goals as
defined by SBOs (Strategic Business Objectives),
CSFs (Critical Success Factors) and KPIs. Business
competencies are then defined and a value chain model
is designed linking processes to each identified
competency. Processes are categorized and results
compared with known relevant reference model, if one
exists, and validated with stakeholders.
3 RESULTS
To be useful, the process reference model for
medication management should be generic enough for
use as a starting point for the development of any
health organization’s process architecture. The
proposed reference model used the process meta-
objects of the BPMO included in LEADing Practice
standards. The reference model created as part of this
research includes one hundred and sixty-four
individual processes categorized in four process areas
and twenty-five process groups. Process Area is
defined as “the highest level of an abstract
categorization of processes”. Process Group is defined
as “a categorization and collection of processes into
common groups”. Process is defined as “a set of
structured activities or tasks with logical behavior that
produce a specific service or product” (von Rosing,
Scheer, & von Scheel, 2014). The process steps and
process activities were not included in the reference
model as this level of detail is context specific at an
organizational and/or department level and could be
different for every organization.
A process can be categorized and tagged according
to the role it fulfils within the organization. The core
(or main processes) are defined as those processes that
provide a service. In the case of medication
management this includes the processes provided at the
point of care. The individual roles involved in main
processes are the clinicians and care providers who
assess, diagnose, prescribe, dispense, administer,
monitor and discharge patients. The support processes
support the delivery of the main processes.
HEALTHINF 2020 - 13th International Conference on Health Informatics
548
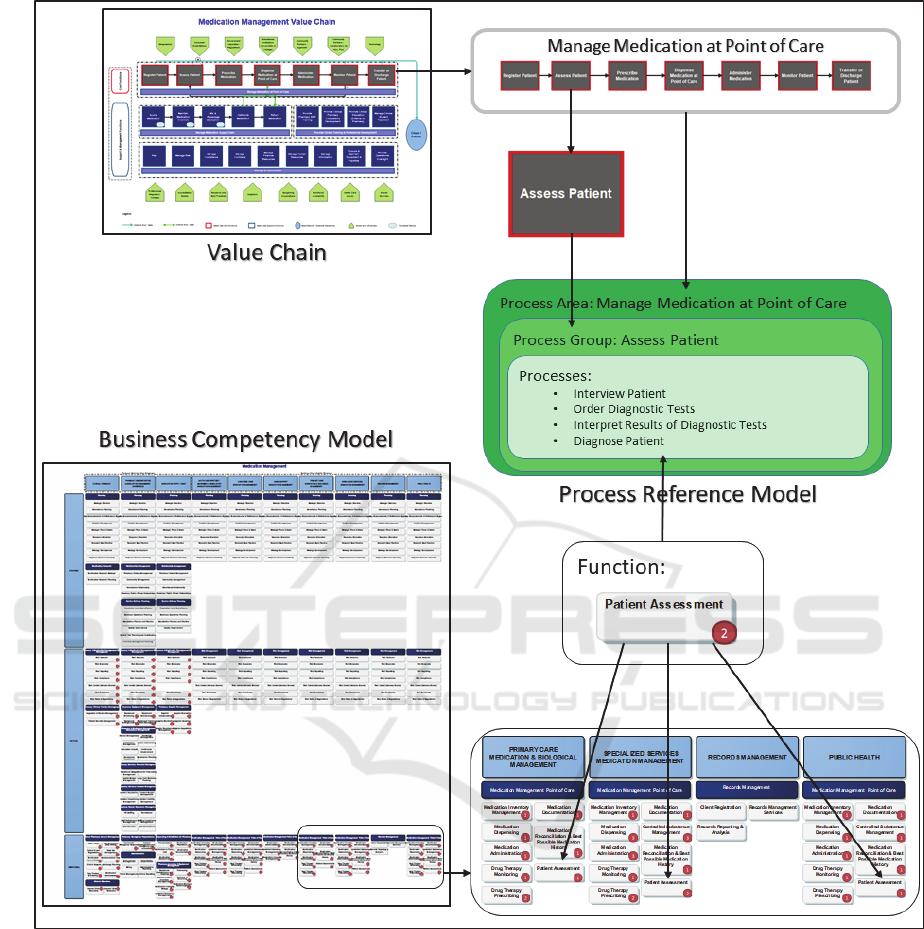
Figure 1: Relationship between Process Reference Model Meta-Objects and Business Artefacts.
In the proposed reference model, the support
processes are categorized into two areas depending on
whether the processes were related to management of
medication supply chain, or provision of training and
education. The recipients of training and education
include both staff and external students who are
placed in the organization as part of their formal
education. A separate grouping of these processes
was deemed appropriate since they could be
considered to be either support or management
processes depending on the recipient. The individual
roles involved in support processes are the pharmacy
staff with respect to medication supply chain and
clinical pharmacist in respect to clinical education.
Management processes include administrative
processes and the processes required to manage the
core and support processes. The process reference
model for medication management further
categorizes these into nine groups based on the
business function. The individual roles involved in
management
processes are the Regional Director,
A Process Reference Model for Enhanced Medication Management
549

Pharmacy Managers, Anti-Microbial Stewardship
Pharmacists, Drug Utilization Pharmacist and
Administrative Assistants.
The Process Areas and the Process Groups used in
the reference model were derived from the Value
Chain. A total of four Process Areas were identified:
1) Manage Medication at Point of Care, 2) Provide
Clinical Training & Professional Development, 3)
Manage Medication Supply Chain, and 4) Manage &
Administrate. In addition, a total of twenty-five
Process Groups were identified. The complete listing
of four Process Areas, twenty-five Process Groups and
one hundred and sixty-four processes is provided in
Appendix 1. Each process is associated with at least
one business competency. The identification of
processes was achieved through review of the
functions included on the Business Competency Model
for Medication Management services.
Each business competency was reviewed and the
processes required to deliver the competency were
listed and included in the process reference model. It
should be noted that a single competency may require
numerous processes to deliver it. Also, some processes
can have a relationship with more than one
competency. An example of this is the process to
“monitor training effectiveness”. This process is
related to more than one competency because they are
separated based on the recipient of the education.
Figure 1 shows the relationship between the
Medication Management Process Reference Model
and the objects included in two business artefacts,
namely, Value Chain and Business Competency
Model.
Two approaches were taken in an effort to validate
the completeness of the process reference model. The
first was to compare the listed processes to those
recorded in other relevant reference models. The
second was to review the initial draft of the process
reference model with the stakeholders, including
Regional Director of Pharmacy, within the host
organization. Two reference models were considered
for the purpose of comparison: 1) SCOR reference
model (Supply Chain Council Inc., 2012) and 2) the
APQC (PCF) Process Classification Framework
(American Productivity and Quality Centre (APQC),
2014). SCOR is specific to supply chain operations and
as such was mainly applicable as a comparator to the
process area of Manage Medication Supply Chain.
SCOR also includes management processes which
were included in the Manage and Administrate process
area. SCOR’s six process groups were mapped to those
included in the reference model. The groups which are
not supply chain specific do not appear in the SCOR
model. The mapping has been excluded from this paper
due to space constraints.
APQC has developed numerous industry specific
Process Classification Frameworks (PCF) including
one related specifically to the provision of health care.
This PCF does not differentiate pharmaceutical
inventory management between centralized inventory
or point of care inventory which have different
processes and specific legislated considerations.
Although it can be used as a general guide it was not
specific enough for use as a medication management
process reference model.
The Regional Director of Pharmacy, the process
owner, reviewed and accepted the process reference
model as a reasonable listing of the processes
associated with medication management and safety.
4 DISCUSSION & CONCLUSION
The proposed Medication Management Process
Reference Model could be employed as a starting point
in other healthcare organizations initiating a process
architecture as it is based on processes which are
relatively standard across healthcare. The processes
are derived from review of the medication
management business competencies included in the
Business Competency Model. These processes are
then categorized into logical groups which are further
categorized into areas.
A common approach to developing a process
architecture is to separate the processes into one of
three types: core, support or management and then
assign these as the highest level of the architecture.
The proposed reference model includes a fourth area
(Clinical Training and Professional Development)
because some processes contained in it could be
considered to be support or management. The clinical
nature of medication education and clinician’s reliance
on education being provided by clinical pharmacists
warranted it as an area on its own. This area also
includes a process group related to training of students
and residents from academic institutions who complete
practicums in the host organization facilities.
The literature review conducted prior to this
research did not reveal a process reference model
specifically for medication management. The methods
and tools used to create the proposed process reference
model could potentially be used to identify processes
in other service areas or indeed across an entire
organization. Further research could include testing of
the proposed model in other healthcare organizations
and also testing and refinement of the methods and
HEALTHINF 2020 - 13th International Conference on Health Informatics
550
tools through application in other service areas or
organizations.
REFERENCES
American Productivity and Quality Centre (APQC). (2014).
apqc process classification framework pcf healthcare
provider members excel. Retrieved November 2014,
from apqc.org.
Au-Yong-Oliveria, M., & Ferreira, J. (2014). World future
review, What if colorful images become more
important than works? Visual represenations as the
basic building blocks of human communication and
dynamic storytelling. World Future Review, 6(1), 48-
54.
Bandra, W., Chand, D., Chircu, A. M., Hintringer, S.,
Karagiannis, D., Recker, J. C., . . . Welke, R. J. (2010).
Business Process Management Education in Academia:
Status, challenges, and recommendations.
Communications of the Association for Information
Systems, 27, pp. 743-776.
Global University Alliance. (2018). Enterprise
Architecture. Retrieved November 2018, from
http://www.globaluniversityalliance.org/research/enter
prise-architecture/
Hendrickx, H. H., Mahakena, S. K., & von Rosing, M.
(2012). The business architecture profession.
Commerce and Enterprise Computing (CED, IEEE
12th Conference. doi:10.1109/CEC.2011.55
Martins, A. C., Giordani, F., & Rozenfeld, S. (2014).
Adverse Drug Events among adult inpatients: a meta
analysis of observational studies. Joournal of Clinical
Pharmacy Therapeutics, 39, 609-620.
Pajik, D., Indihar-Stemberger, M., & Kovacic, A. (2012).
Reference model design: An approach and its
application. IEEE, (pp. 455-460).
Ponsignon, F., Smart, P., & Maul, R. (2012). Process design
principles in service firms: Universal or context
dependent? A literature review and new research
directions. 23(11), 1273-1296.
Singh, K. (n.d.). Integration of Intravenous Infusion pumps
and RFID/NFC-safeguard: the NextGen Infusion
Pumps. Retrieved December 30, 2019, from
http://ks.kanadasoft.ca/pumps
Supply Chain Council Inc. (2012). Supply Chain
Operations Reference Model Revision 11.0. Retrieved
November 2017, from www.supply-chain.org.
von Rosing, M., Laurier, W., & Polovina, S. (2014). The
BPM Ontology. In M. von Rosing, M. Scheer, & H.
Scheel, The Complete Business Process Handbook,
Body of Knowledge from Process Modeling to BPM
Volume 1 (pp. 102-107). USA: Morgan Kaufmann
Publishers Inc.
von Rosing, M., Scheer, A. W., & von Scheel, H. (2014).
The Complete Business Process Handbook Body of
Knowledge from Process Modeling to BPM (Vol. 1).
USA: Morgan Kaufmann.
APPENDIX 1: THE REFERENCE MODEL
1 Manage Medication at Point of Care
1.1 Register Patient
1.1.1 Enrol Patient in Appropriate Information
S
y
ste
m
1.1.2 Confirm Patient Identification and
Identif
y
for Clinical Pharmac
y
Services
1.2 Assess Patient
1.2.1 Interview Patien
t
1.2.2 Order Dia
g
nostic Tests
1.2.3 Interpret Results of Dia
g
nostic Tests
1.2.4 Dia
g
nose Patien
t
1.3 Prescribe Medication
1.3.1 Conduct Best Possible Medication
Histor
y
Interview
1.3.2 Conduct Medication Reconciliation
1.3.3 Order Medication
1.3.4 Tria
g
e medication orders
1.3.5 Perform Clinical assessment of
medication orde
r
1.4 Dispense Medication at Point of Care
1.4.1 Maintain Point of Care Inventor
y
1.4.2 Mana
g
e Patient Owned Medications
1.4.3 Dispense Medication
1.5 Administer Medication
1.5.1 Prepare medication if require
d
1.5.2 Administer Medication to Patients
1.5.3 Complete Medication Administration
Recor
d
1.6 Monitor Patient
1.6.1 Provide Pharmaceutical Care to Patients
1.6.2 Deliver Patient Education on Medication
Therap
y
1.6.3 Monitor Patient Response to Medication
Therap
y
1.7 Transfer or Discharge Patient
1.7.1 Plan for Patient Dischar
g
e
1.7.2 Dischar
g
e Patien
t
2 Provide Clinical Training & Professional
Development
2.1 Provide Pharmacy Staff Training
2.1.1 Identif
y
Trainin
g
Needs
2.1.2 Develop Trainin
g
Materials
2.1.3 Deliver Trainin
g
A Process Reference Model for Enhanced Medication Management
551

2.1.4 Monitor Learner Progress and Provide
Feedbac
k
2.1.5 Monitor Trainin
g
Effectiveness
2.2 Provide Clinical Pharmacy Competency
Development
2.2.1 Establish Clinical Pharmacy
Competencies
2.2.2 Develop Competenc
y
Evaluation
2.2.3 Develop Competenc
y
Trainin
g
2.2.4 Deliver Clinical Pharmac
y
Trainin
g
2.2.5 Monitor Learner Progress & Provide
Feedbac
k
2.2.6 Monitor Trainin
g
Effectiveness
2.3 Provide Clinical Education(External to
Pharmacy)
2.3.1 Identif
y
Trainin
g
Needs
2.3.2 Develop Trainin
g
Materials
2.3.3 Deliver Trainin
g
2.3.4 Monitor Learner Progress and Provide
Feedbac
k
2.3.5 Monitor Trainin
g
Effectiveness
2.4 Manage Clinical Student Placement
2.4.1 En
g
a
g
e with Education Providers
2.4.2 Identify Potential Candidates & Make
Selection
2.4.3 Develop Trainin
g
Plan
2.4.4 Monitor Student Progress & Provide
Feedbac
k
2.4.5 Evaluate Effectiveness of Trainin
g
Plan
3 Manage Medication Supply Chain
3.1 Source Medication
3.1.1 Establish & Maintain Supplier
Requirements
3.1.2 Evaluate and Approve Potential Suppliers
3.1.3 Identif
y
and Maintain Supplier Lis
t
3.1.4 Ne
g
otiate with Suppliers
3.1.5 Collaborate with Suppliers
3.1.6 Monitor Supplier Performance
3.1.7 Perform Analysis and Response to Drug
Shorta
g
es
3.1.8 Purchase Medication
3.1.9 Receive purchased medication
3.1.10 Initiate Pa
y
men
t
3.1.11 Pa
y
Suppliers
3.2 Maintain Medication Inventory
3.2.1 Define Inventor
y
Strate
g
ies
3.2.2 Define Inventor
y
Deman
d
3.2.3 Create Inventor
y
Plan
3.2.4 Define Performance Metrics
3.2.5 Establish Standards for Medication
Stora
g
e
3.2.6 Ship Inventory to secondary inventory
locations
3.2.7 Store purchased medication
3.2.8 Monitor medication stora
g
e procedures
3.2.9 Perform Inventor
y
Coun
t
3.3 Mix & Repackage Medication
3.3.1 Issue components from inventor
y
3.3.2 Compound Medications Outside Laminar
Hoo
d
3.3.3 Compound Medications within Laminar
Hoo
d
3.3.4 Packa
g
e Compounded Medication
3.3.5 Add Compounded Medications to
Inventor
y
3.3.6 Remove bulk packaged goods from
inventor
y
location
3.3.7 Repacka
g
e into sin
g
le use doses
3.3.8 Restore unit dose packa
g
es in inventor
y
3.3.9 Repackage medications for 24 hour Batch
( patient specific)
3.3.10 Pick medication from inventor
y
3.3.11 Issue medication from perpetual
inventor
y
3.4 Distribute Medication
3.4.1 Transport purchased medication to
secondar
y
inventor
y
location
3.4.2 Document Patient Specific Medication
Orders
3.4.3 Verif
y
Patient Specific Medication Orders
3.4.4 Dispense Patient Specific Medication
3.4.5 Replenish Ward Stoc
k
3.5 Return Medication
3.5.1 Identify expired medications in Pharmacy
Inventor
y
3.5.2 Return Expired Dru
g
s to Pharmac
y
3.5.3 Return Expired Drugs eligible for refund
to Suppliers
3.5.4 Dispose of expired controlled substances
3.5.5 Dispose of expired medications
4 Manage & Administrate
4.1 Plan
4.1.1 Develop Strate
g
ic Plan & Goals
4.1.2 Establish Portfolio Priorities
4.1.3 Establish Governance Plans
4.1.4 Allocate Resources
4.1.5 Develop Strategy for Pharmacy &
Medication Mana
g
emen
t
4.1.6 Establish Population Level Surveillance
Plan
4.1.7 Develop & Communicate Regional
Services Plan
4.1.8 Plan Research Strate
gy
HEALTHINF 2020 - 13th International Conference on Health Informatics
552

4.1.9 Develop Medication Research Strate
gy
4.1.10 Establish Formular
y
Mana
g
ement Plan
4.1.11 Establish Business Operatin
g
Plan
4.1.12 Establish Standards for Pharmacy &
Medication Mana
g
emen
t
4.1.13 Establish Plan for Clinical Trials
4.1.14 Identif
y
Qualit
y
Improvement Plan
4.1.15 Plan for lon
g
term business operations
4.1.16 Establish Performance Measurement
Plan
4.1.17 Maintain Dru
g
Formular
y
4.2 Manage Risk
4.2.1 Establish Risk Anal
y
sis Framewor
k
4.2.2 Establish Risk Measures
4.2.3 Establish Risk Monitorin
g
Plan
4.2.4 Establish Risk Reportin
g
4.2.5 Establish & Maintain Response to
Adverse Events
4.2.6 Establish & Maintain Risk Rules &
Re
g
ulations
4.2.7 Establish & Mana
g
e Risk Compliance
4.3 Monitor Compliance
4.3.1 Manage and Monitor Progress related to
Governance Plan
4.3.2 Establish Compliance Plan and Reportin
g
4.3.3 Validate medication orders adhere to Safe
Medication Order Writin
g
4.3.4 Document receipt, administration and
disposal of controlled substances
4.3.5 Document receipt, administration and
disposal of controlled substances
4.3.6 Monitor & Control compliance with
Policies & Procedures
4.3.7 Evaluate & Audit effectiveness of
Policies & Procedures
4.4 Manage Contracts
4.4.1 Ne
g
otiate contracts
4.4.2 Mana
g
e contract Bud
g
e
t
4.4.3 Evaluate and monitor contract liabilit
y
4.4.4 Evaluate and monitor contract
p
erformance and compliance
4.5 Manage Human Resources
4.5.1 Identif
y
Human Resource Requirements
4.5.2 Recruit Staff to meet Identified Needs
4.5.3 Recruit Staff to identified Needs
4.5.4 Orientate Staff
4.5.5 Schedule Staff
4.5.6 Mana
g
e Staff Performance
4.5.7 Mana
g
e Staff Reco
g
nition Pro
g
ra
m
4.6 Manage Financial Resources
4.6.1 Identif
y
Operatin
g
Bud
g
et Requirements
4.6.2 Monitor Operatin
g
Expenditures
4.6.3 Initiate Operating Budget Remediation
Actions
4.6.4 Identif
y
Capital Bud
g
et Requirements
4.6.5 Monitor Capital Expenditures
4.6.6 Initiate Capital Budget Remediation
Actions
4.6.7 Forecast operating and capital budget
p
erformance
4.6.8 Initiate Accounts Receivable
4.6.9 Collect Accounts Receivable
4.6.10 Mana
g
e Emplo
y
ee Travel Expenses
4.7 Manage Information
4.7.1 Establish Information S
y
stem Standards
4.7.2 Mana
g
e Information S
y
stem Access
4.7.3 Mana
g
e Patient Records
4.7.4 Monitor & Improve Data Qualit
y
4.7.5 Maintain clinical pharmac
y
patient recor
d
4.7.6 Maintain Medication information
resources
4.7.7 Maintain Inventor
y
Data
4.7.8 Manage Master Drug Library for Infusion
Pumps
4.7.9 Develop and Maintain medication order
sets
4.7.10 Develop & Maintain Performance
Monitorin
g
Reports
4.8 Procure & Maintain Equipment &
Facilities
4.8.1 Establish & Mana
g
e Equipment Schedule
4.8.2 Procure Equipment through Renting or
Leasin
g
Option
4.8.3 Establish & Manage Plan for Equipment
Maintenance
4.8.4 Establish & Maintain Asset Tracking
Policies & Procedures
4.9 Provide Operational Oversight
4.9.1 Establish and Mana
g
e Partnerships
4.9.2 Establish & Manage Provincial
Government Relationship
4.9.3 Establish & Manage Municipal
Government Relationships
4.9.4 Establish & Manage Public Private
Partnerships
4.9.5 Establish & Staff Pharmacy Hours of
Operation
4.9.6 Promote continuous Improvemen
t
4.9.7 Implement and Maintain Anti-Microbial
Stewardship Pro
g
ra
m
4.9.8 Promote Integration & Practice
Mana
g
emen
t
4.9.9 Develop & Maintain Community &
Emergency Response Plan
4.9.10 Research Best Practice
A Process Reference Model for Enhanced Medication Management
553

4.9.11 Establish Emergency Response Policies
& Procedures
4.9.12 Mana
g
e Fleet Vehicles
4.9.13 Develop & Monitor Administrative
Reports
4.9.14 Develop & Monitor Clinical Reports
4.9.15 Develop & Publish Performance Reports
HEALTHINF 2020 - 13th International Conference on Health Informatics
554
