
Preventing Spin Relaxation of Optically Pumped Alkali Metal Atoms
in Magnetometer by Atomically Thin Film Coating
H. Kumagai
1
, R. Yoshimitsu
2
, S. Takeda
1
, E. Ogawa
1
, T. Kosuge
1
, H. Ishikawa
2
, T. Sato
3
and M. Suzuki
3
1
School of Allied Health Sciences, Kitasato University, 1-15-1 Kitasato, Minami, Sagamihara, 252-0373, Japan
2
School of Science, Kitasato University, 1-15-1 Kitasato, Minami, Sagamihara, 252-0373, Japan
3
J. A. Woollam Japan Corp, Fuji 2F 5-22-9 Ogikubo, Suginami-ku Tokyo, 167-0051, Japan
Keywords: Atomic Layer Deposition, Optically Pumped Atomic Magnetometer, Spin Polarization.
Abstract: We developed molecular layer deposition method of atomically thin hybrid polymer film for the first time by
developing atomic layer deposition method with sequential surface chemical reactions in order to minimize
the effect of the dipole-dipole interaction between the electron spin of alkali metal atoms and the nuclear spin
of the atoms in the glass of the cell. We controlled film thickness of polymer thin film precisely and finally
aimed at improving the sensitivity of the optically pumped atomic magnetometer. In the presentation, we
report on the relaxation time of spin polarization by atomically thin hybrid polymer film with laser pump-
probe method.
1 INTRODUCTION
The magneto-cardiogram test, which measures a very
small magnetic field generated from the human heart
with a highly sensitive magnetic sensor and performs
two-dimensional mapping analysis, is expected to be
able to evaluate cardiac electrical activity with higher
spatial resolution and higher sensitivity than the
electrocardiogram method in principle. On the other
hand, extremely weak magnetic field measurement of
fT order generated from neurons of the cerebral
cortex is expected to play a very important role in
noninvasively investigating human cranial nerve
activity. At present, a superconducting quantum
interference device (SQUID) having a sensitivity of 1
fT / Hz
1/2
order is used as a magnetic sensor for
magneto-cardiography measurement and magneto-
encephalography measurement. Using SQUID makes
it possible to acquire knowledge about magnetic field
mapping from the heart and brain and basic electrical
activity in vivo, but since SQUID uses
superconducting quantum interference effect, liquid
helium It is necessary to operate it in an extremely
low temperature state, and there is a problem that a
large-sized apparatus becomes expensive
maintenance cost. In recent years, attention has been
paid to an optical pumping atomic magnetometer
which measures a magnetic field by using spin
polarization of alkali-metal atoms through the optical
pumping method. The optical pumping method is a
method in which light is used to create a large
difference in the occupation number of atoms at two
close energy levels and the optically pumped alkali
metal atoms are spin polarized and the magnetic field
applied there is linear. In order to rotate the plane of
polarization of polarized light, we can estimate the
magnetic field at room temperature from this angle of
rotation. It has been energetically studied in the
United States, Europe and the like, and at the National
Institute of Standards and Technology (NIST), it has
become compact to the size of a chip scale small
watch (Schwindt et al., 2004).
In the optically pumped atomic magnetometer, the
rotation angle of the polarization plane of the probe
light becomes more sensitive to the change of the
magnetic field as the relaxation rate of the spin
becomes smaller. Recent reports from Princeton
University reported that sensor sensitivity can reach
sub fT / Hz
1/2
order by using SERF (Spin - Exchange
- Relaxation - Free) state where the relaxation rate of
spin polarization decreases (Kominis et al., 2003).
Optical pumping atomic magnet sensors operating in
the SERF state are expected. Therefore, it is
extremely important to suppress the relaxation rate of
spin polarization to a small value when spin-polarized
atoms are used in an alkali vapor cell in order to
measure with high precision. Although polarized spin
is relaxed by collision of atoms against the inner wall
250
Kumagai, H., Yoshimitsu, R., Takeda, S., Ogawa, E., Kosuge, T., Ishikawa, H., Sato, T. and Suzuki, M.
Preventing Spin Relaxation of Optically Pumped Alkali Metal Atoms in Magnetometer by Atomically Thin Film Coating.
DOI: 10.5220/0009161302500253
In Proceedings of the 13th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2020) - Volume 1: BIODEVICES, pages 250-253
ISBN: 978-989-758-398-8; ISSN: 2184-4305
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
of the cell, relaxation of spin polarization due to
collision of this atom with the inner wall of the cell
can be suppressed by coating the inner wall of the
cell. This coating is called a spin relaxation
preventing coating, and paraffin (CH
3
(C
n
H
2n
) CH
3
:
n> 20) has been widely used so far. The effect of
prevention of spin relaxation by paraffin coating was
first demonstrated by Robinson et al. in 1958
(Robinson et al., 1958). With no relaxation of spin
polarization maximum of about 10,000, it is possible
to collide with the inner wall, and it is expected to be
applied to the field of ultrahigh sensitivity magnetic
sensors and quantum communication. The well-
known paraffin coating on the inner wall surface of
the cell can be said to be an extremely useful means
for obtaining a long spin relaxation time, but the
physical action of its spin relaxation prevention effect
is not well understood at present. According to
Bouchiat et al. (Bouchiat et al., 1966), dipole-dipole
interaction between electron spin of spin-polarized
atom and nuclear spin of coated surface hydrogen
atom, or orbital angular momentum and electron spin
of relative motion between coating surface atoms and
spin-polarized atoms Interaction is said to be the
cause of spin relaxation. However, the result of this
research alone is insufficient to understand the
workings of the coating. For example, an effect of
preventing spin relaxation at a high temperature is
dulled, annealing at 80 °C for several hours in the
presence of alkaline vapor called "aging process"
after coating of paraffin increases spin relaxation
prevention effect (Seltzer et al., 2010), but neither has
been fully understood. Spin relaxation prevention
effect obtained by the same coating material and the
same manufacturing method is greatly different, and
it falls within the skill of coating applicants and
researchers.
One of authors has independently developed an
atomic layer deposition method and an atomic layer
deposition method of oxide by sequential surface
chemical reaction using organometallic gas and water
vapor as a starting material, so that an alkyl group (n
= 1, 2, 3). We found that precise film thickness
control can be realized using "self-limiting
mechanism" appearing in the adsorption process, and
in particular, we have found that it is possible to
realize precise film thickness control using "self-
limiting mechanism", to overcome the extremely
difficult task of making a multilayer film structure
(Kumagai et al., 1997).
2 EXPERIMENTAL SETUP
The experimental setup was comprised of a stainless
steel vacuum chamber with two computer-controlled
leak valves, a capacitor manometer, turbo-molecular
pump (TMP) and quartz crystal unit to allow in situ
measurements during growth of metal oxides. As a
substrate, (100)-oriented Si wafers were used together
with quartz glass cells. The substrate was first
ultrasonically cleaned in conventional organic
solvents, then dipped in 4.7% HF to remove the native
oxide. After rinsing it in overflowing deionized water,
it was loaded into a vacuum chamber. As vapor
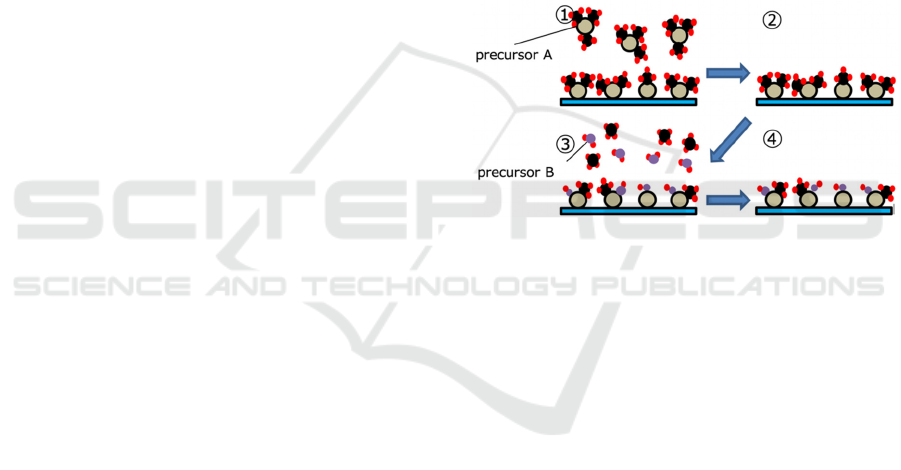
sources for the aluminum oxide film, two precursors
were used in Fig.1. high-purity trimethyl-aluminum
(TMA) and ethanol (EtOH) were used as precursor A
and B, respectively.
Figure 1: Atomic layer deposition utilizing two distinct
precursors (A, B) sequentially dosed to the substrate
producing a chemical reaction.
EtOH was prepared by Ethanol JIS special grade,
≥99.5%.These vapors were introduced alternately by
two computer-controlled leak valves into the chamber
which was evacuated by a TMP to a pressure below
10
-7
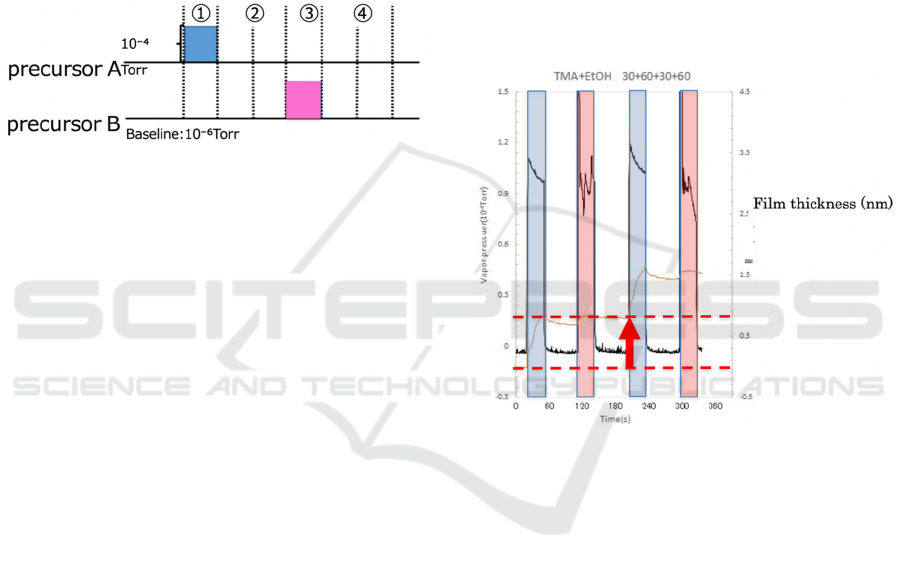
Torr. Figure 2 shows input signals applied to the
computer-controlled leak valves for generating each
vapor pulse whose peak vapor pressure reached 1x10
-
4
Torr. The duration of supplying each vapor pulse
was 30 s, while the chamber was continuously
exhausted during the growth. The time point exactly
20 s before the first dosing of TMA vapor was defined
as t= 0 s. Therefore, TMA vapor was first introduced
at t = 20 s during the supplying time, and then at t=
110 s, EtOH vapor was first introduced, and then at
t= 200 s, TMA vapor was introduced again. From t =
290 s onwards, these binary vapors were supplied
alternately according to the sequence in Fig. 2.
Atomic layer deposition at room temperature was
carried out by changing the combination of
trimethylaluminum (TMA) and water vapor (H
2
O)
which was often used in the atomic layer deposition
method, in addition to H
2
O as ethanol as an oxidizing
Preventing Spin Relaxation of Optically Pumped Alkali Metal Atoms in Magnetometer by Atomically Thin Film Coating
251
agent. Assuming that the introduction cycle period is
6 s, TMA was introduced to the first 0 - 1 s and an
oxidizing agent was introduced to 3 - 4 s. The peak
pressure of the material gas to be introduced was kept
constant at 10
-4
Torr, the total time of introduction of
the source gas was kept constant, the introduction
pulse time and the number of cycles were changed to
seven types, and the deposition characteristics were
investigated. All atomic layer deposition processes
were done at room temperature and a thin film was
deposited on the substrate. The film thickness and the
refractive index were measured with a spectroscopic
ellipsometer.
Figure 2: Input signals applied on the computer-controlled
leak valves for generating each vapor pulse.
3 MOLECULAR LAYER
DEPOSITION OF
ORGANIC-INORGANIC
HYBRID POLYMER THIN
FILM REALIZED
Figure 3 shows variations of vapor pressure in the
vacuum chamber and film thickness with growth time
at room temperature. The vapor pressures in the
vacuum chamber evacuated by a TMP to a pressure
below 10
-7
Torr follows the input signals in Fig. 2.
Duration of each pulse of vapor pressure was 30 s.
The film thickness also shown in Fig. 3 indicates the
thickness from the initial surface (t = 0 s). The
duration of supply of TMA and EtOH vapors which
were introduced in the sequence shown in Fig. 3, was
30 s. Figure 3 shows the case when binary vapors of
TMA and EtOH were supplied by taking into account
the sequence shown in Fig. 2, an increase of around 1
nm is found to occur upon the introduction of TMA.
This is because dosing of TMA to surface -O-H
groups causes chemical reactions by which OH
groups change into -O-Al-CH
3
groups, whereas
dosing of EtOH to surface Al- CH
3
, groups causes
chemical reactions in which Al- CH
3
groups change
into Al-O-H groups. Although actual surface
reactions must be more complicated than the
simplified picture mentioned here, the picture can
also be supported by infrared spectroscopic studies.
Modification of O-H-terminated surfaces to -O-
Al-CH
3
makes the increase of thickness 0.1 nm
greater than modification of Al-CH
3
-terminated
surfaces to Al-O-H. It was found in Fig. 3 that the film
thickness slightly decreased just after the increase,
because high vacuum caused desorption of molecules
which had adsorbed at room temperature. Growth
rates were 0.887 nm/cycle, the same as obtained by
dividing the total thickness of the film from an ex situ
variable-angle spectroscopic ellipsometer (LA.
Woollam Co., Inc.) by the number of growth cycles.
This exhibits characteristics of self-limiting nature of
adsorption which are characteristic of the growth
technology in this study.
Figure 3: Variations of vapor pressure in the vacuum
chamber and film thickness with growth time at room
temperature.
4 MEASURE SPIN
POLARIZATION
RELAXATION TIME
To characterize the quality of the film fabricated on
inner-wall of the glass cell, we performed
measurements of the relaxation time of the optical
pumped rubidium atoms with the pump probe method
in Fig.4. The laser frequency of the pump and probe
light beams was same and then tuned to be resonant
to 5S
1/2
(F = 3) -> 5P
3/2
(F' = 2, F' = 3, F' = 4) of
85
Rb
optical transition, until a maximum of fluorescent
intensity in the separate, uncoated cell was obtained.
Then, the pump beam in the cell was supplied by
abrupt opening of a shutter. The atoms on the Rb atom
ground state 5S
1/2
(F = 3) were excited by the
BIODEVICES 2020 - 13th International Conference on Biomedical Electronics and Devices
252
radiation; and the radiation populated 5S
1/2
(F = 2)
ground state through the intermediate atomic upper
states 5P
3/2
(F' = 1, F' = 2, F' = 3, F' = 4). As a result
of the optical pumping process, the amplitude of 5S
1/2
(F = 3) line decreased while the amplitude of 5S
1/2
(F
= 2) line increased.
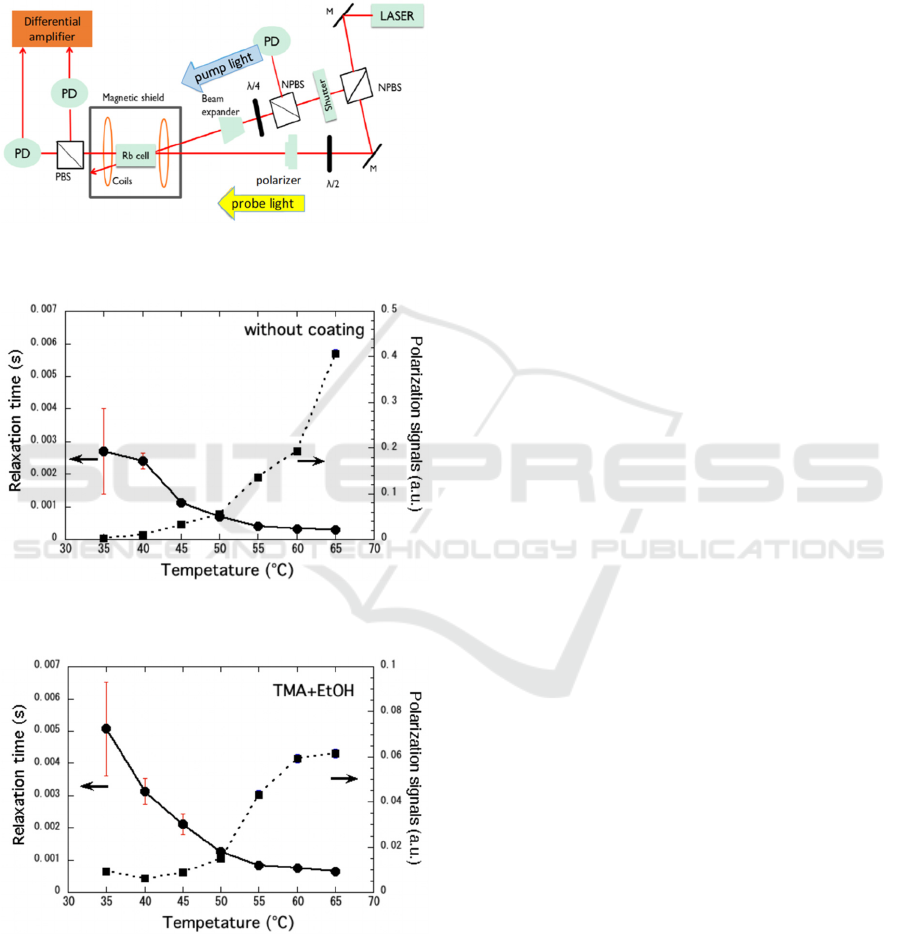
Figure 4: Schematic of the setup for spin relaxation
measurement.
Figure 5: Temperature dependencies of spin relaxation time
and polarization signal in a Rb cell without coating.
Figure 6: Temperature dependencies of spin relaxation time
and polarization signal in a Rb cell with thin film coated by
sequentially dosing TMA and ethanol vapor.
Figure 5 shows temperature dependencies of spin
relaxation time and polarization signal in a Rb glass
cell without coating. At 35°C, the relaxation time was
estimated as 2.7 ms. In a Rb cell with thin film coated
by sequentially dosing TMA and ethanol vapor,
temperature dependencies of spin relaxation time and
polarization signal are shown in Fig. 6.
The relaxation time in the glass cell with coating
was higher than those without coating. At 35°C, it
was more than 5 ms, twice higher than that without
coating.
5 CONCLUSIONS
We controlled film thickness of hybrid polymer thin
film precisely by developing atomic layer deposition
method with sequential surface chemical reactions
and then could improve the relaxation time of spin
polarization by coating with the thin film. Although
the physical action of its spin relaxation prevention
effect is not well understood at present, it will
definitely improve the sensitivity of the optically
pumped atomic magnetometer.
REFERENCES
Schwindt, P. D. D., Knappe, S., Shah, V., Hollberg, L.,
Kitching, J., Liew, L. A., and Moreland, J. (2004).
Chip-scale atomic magnetometer. Appl. Phys. Lett.,
85(26): 6409–6411.
Kominis, I. K., Kornack, T. W., Allred, J. C., and Romalis,
M. V. (2003). A subfemtotesla multichannel atomic
magnetometer. Nature, 422: 596–599.
Robinson, H.G., Ensberg, E.S., and Dehmelt, H.G. (1958).
Preservation of spin state in free atom-inert surface
collisions. Bull. Am. Phys. Soc., 3: 9.
Bouchiat, M. A. and Brossel, J. (1966). Relaxation of
Optically Pumped Rb Atoms on Paraffin-Coated Walls.
Phys. Rev., 147(1): 41–54.
Seltzer, S. J., Michalak, D. J., Donaldson, M. H., Balabas,
M. V., Barber, S. K., Bernasek, S. L., Bouchiat, M.-A.,
Hexemer, A., Hibberd, A. M., Jackson Kimball, D. F.,
Jaye, C., Karaulanov, T., Narducci, F. A., Rangwala, S.
A., Robinson, H. G., Shmakov, A. K., Voronov, D. L.,
Yashchuk, V. V., Pines, A., and Budker, D., (2010).
Investigation of antirelaxation coatings for alkali-metal
vapor cells using surface science techniques. J. Chem.
Phys., 133(144703): 1–11.
Kumagai, H., Toyoda, K., Kobayashi, K., Obara, M., and
Iimura, Y. (1997). Titanium oxide/aluminum oxide
multilayer reflectors for “water-window” wavelengths.
Appl. Phys. Lett., 70: 2338-2340.
Preventing Spin Relaxation of Optically Pumped Alkali Metal Atoms in Magnetometer by Atomically Thin Film Coating
253
